PHR1709
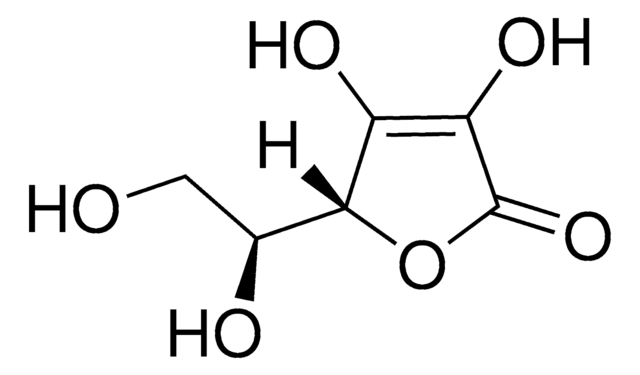
Ascorbic Acid Impurity D
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Methyl-D-Sorbosonate (Ascorbic Acid Imp D), Methyl-D-Sorbosonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001170
API family
ascorbic acid
CofA
current certificate can be downloaded
packaging
ampule of 30 mg
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Ascorbic acid Impurity D is an impurity of ascorbic acid (AA), which is also known as vitamin C. It is found in plant tissues and exhibits antioxidant property.
Application
Ascorbic acid may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using automatic potentiometric flow titration method.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Analysis Note
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Other Notes
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Footnote
To see an example of a Certificate of Analysis for this material enter LRAA7297 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
related product
Product No.
Description
Pricing
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Automatic potentiometric flow titration procedure for ascorbic acid determination in pharmaceutical formulations
Paim SPA, et al.
Journal of Pharmaceutical and Biomedical Analysis, 28(6), 1221-1225 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service