PHR1645
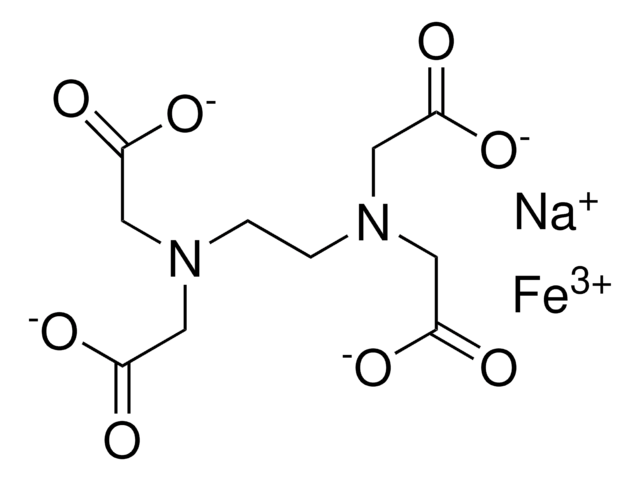
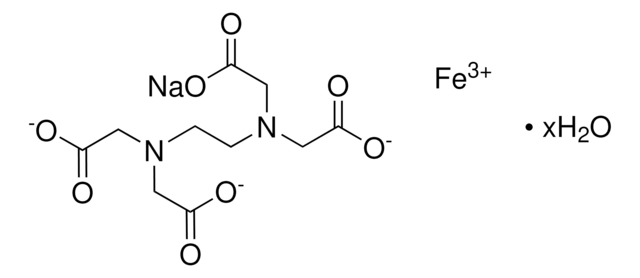
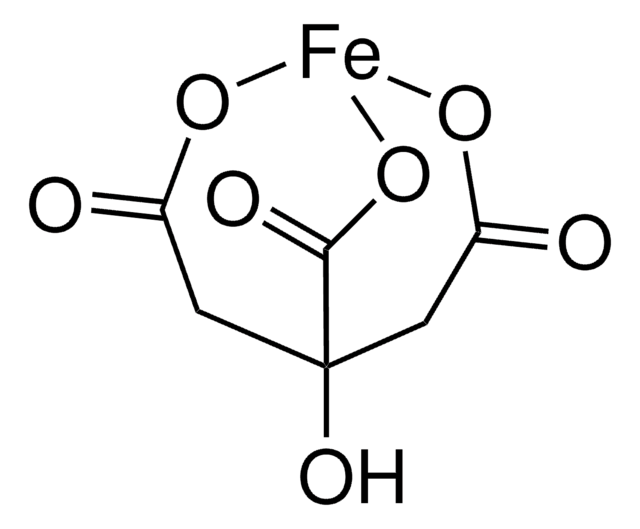
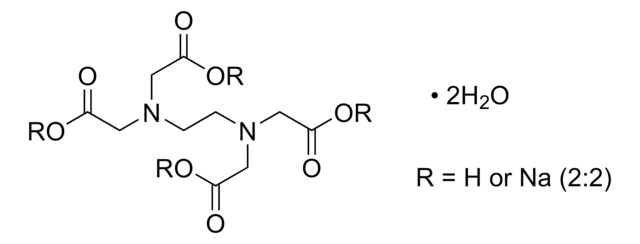
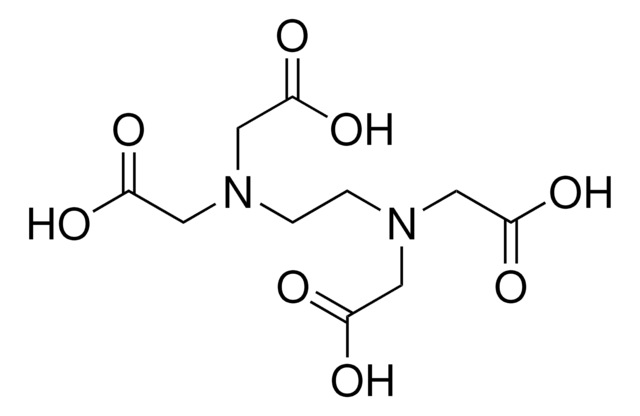
Sodium Iron EDTA
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Ethylenediaminetetraacetic acid iron(III) sodium salt trihydrate
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1614239
API family
sodium iron edta
form
powder
CofA
current certificate can be downloaded
packaging
pkg of 1 g
application(s)
pharmaceutical
storage temp.
2-30°C
InChI
1S/C10H16N2O8.Fe.Na/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20;;/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20);;/q;+3;+1/p-4
InChI key
MKWYFZFMAMBPQK-UHFFFAOYSA-J
General description
Application
Analysis Note
Other Notes
Footnote
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service