H0200000
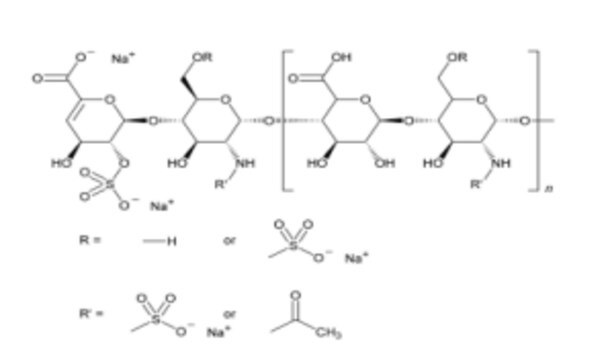
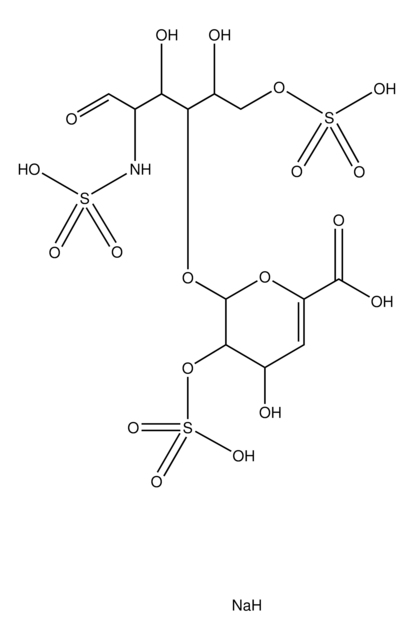
Heparin sodium
BRP, European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
heparin
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
Looking for similar products? Visit Product Comparison Guide
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the Authority of the Issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Heparin sodium EP reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia. Their suitability for any other use is not guaranteed and is the sole responsibility of the user. This standard is not intended for human or animal use.
Established for use in the:
Established for use in the:
- Assay of unfractionated heparin according to the Ph. Eur. General text 2.7.5. with assigned contents of 985 IU/mL for anti-IIa and 995 IU/mL for anti-Xa activities
- Test for heparin in human antithrombin III according to the Ph. Eur. Monograph 0878 with an assigned content of 1035 IU/mL for sheep plasma clotting assay
- Assay of protamine sulfate according to the prescriptions of the Ph. Eur. Monograph 0569
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Assay of Heparin
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 2201-2202 null
Vanja Cnops et al.
Journal of biomedical materials research. Part A, 108(12), 2473-2483 (2020-05-18)
Neurons of the central nervous system do not regenerate spontaneously after injury. As such, biofunctional tissue scaffolds have been explored to provide a growth-promoting environment to enhance neural regeneration. In this regard, aligned electrospun fibers have proven invaluable for regeneration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service