89483
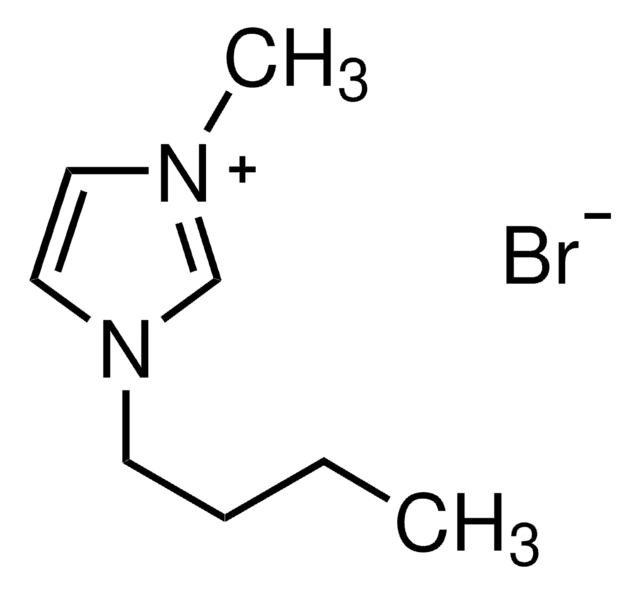
1-Ethyl-3-methylimidazolium bromide
≥97.0% (T)
Synonym(s):
1-Ethyl-3-methyl-1H-imidazolium bromide, 1-Methyl-3-ethylimidazolium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11BrN2
CAS Number:
Molecular Weight:
191.07
Beilstein:
5162084
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Quality Level
Assay
≥97.0% (T)
form
crystals
SMILES string
[Br-].CCn1cc[n+](C)c1
InChI
1S/C6H11N2.BrH/c1-3-8-5-4-7(2)6-8;/h4-6H,3H2,1-2H3;1H/q+1;/p-1
InChI key
GWQYPLXGJIXMMV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
1-Ethyl-3-methylimidazolium bromide can be used to prepare:
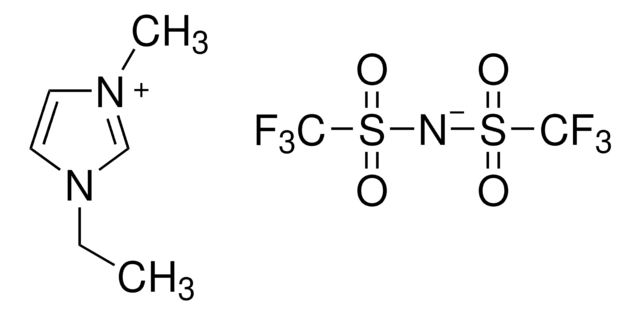
- 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide
- 1-ethyl-3-methylimidazolium tetrafluoroborate
- (EMI)[Cd(BTC)] (EMI = 1-ethyl-3-methylimidazolium, BTC = 1,3,5-benzenetricarboxylate), a coordination polymer
Other Notes
Hydrophobic, highly conductive imidazolium molten salt melting at ambient temp.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Voltammetry of oxygen in the room-temperature ionic liquids 1-ethyl-3-methylimidazolium bis ((trifluoromethyl) sulfonyl) imide and hexyltriethylammonium bis ((trifluoromethyl) sulfonyl) imide: one-electron reduction to form superoxide. Steady-state and transient behavior......
Buzzeo MC, et al.
The Journal of Physical Chemistry A, 107(42), 8872-8878 (2003)
The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals.
J. Chem. Soc., Dalton Trans., 13, 2133-2140 (1999)
Ionic liquid as solvent for the synthesis and crystallization of a coordination polymer:(EMI)[Cd(BTC)](EMI=1-ethyl-3-methylimidazolium,BTC=1,3,5 benzenetricarboxylate).
Liao JH, et al.
Crystal Growth & Design, 6(5), 1062-1063 (2006)
Seungmin Oh et al.
The journal of physical chemistry. B, 123(39), 8274-8284 (2019-09-11)
Ionic liquids with aprotic heterocyclic anions (AHAs) have been developed for CO2 capture but have been considered for other applications as well. Previously, we have shown that AHA ILs, where the only site for reaction with CO2 is the anion
Pierre Bonhôte et al.
Inorganic chemistry, 35(5), 1168-1178 (1996-02-28)
New, hydrophobic ionic liquids with low melting points (<-30 degrees C to ambient temperature) have been synthesized and investigated, based on 1,3-dialkyl imidazolium cations and hydrophobic anions. Other imidazolium molten salts with hydrophilic anions and thus water-soluble are also described.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service