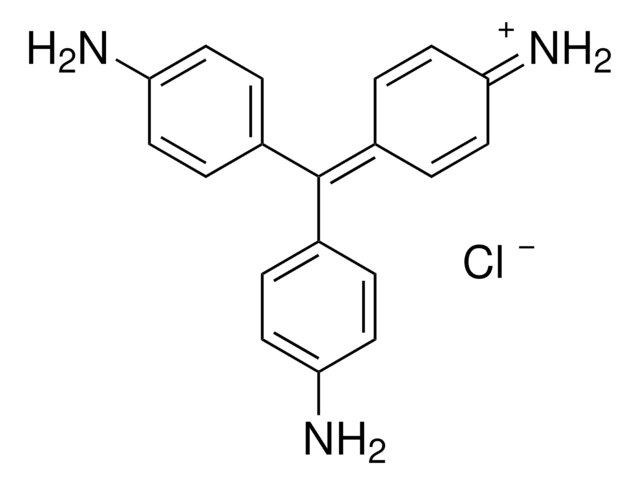
857343
Basic Fuchsin
certified by the Biological Stain Commission, Dye content ≥88 %
Synonym(s):
Basic Parafuchsin, Basic Red 9, Magenta™ O, Parafuchsin hydrochloride, Paramagenta hydrochloride, Pararosaniline chloride, Pararosaniline hydrochloride
About This Item
Recommended Products
grade
certified by the Biological Stain Commission
Quality Level
form
powder
composition
Dye content, ≥88%
color
green to dark green
pH
1.0-3.1, purple to red
mp
268-270 °C (dec.) (lit.)
λmax
544 nm
ε (extinction coefficient)
≥11000 at 235-239 nm in 50% ethanol at 0.003 g/L
≥17000 at 287-291 nm in 50% ethanol at 0.003 g/L
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
[Cl-].Nc1ccc(cc1)C(\c2ccc(N)cc2)=C3/C=CC(=[NH2+])C=C3
InChI
1S/C19H17N3.ClH/c20-16-7-1-13(2-8-16)19(14-3-9-17(21)10-4-14)15-5-11-18(22)12-6-15;/h1-12,20H,21-22H2;1H
InChI key
JUQPZRLQQYSMEQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Suitability
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service