60018
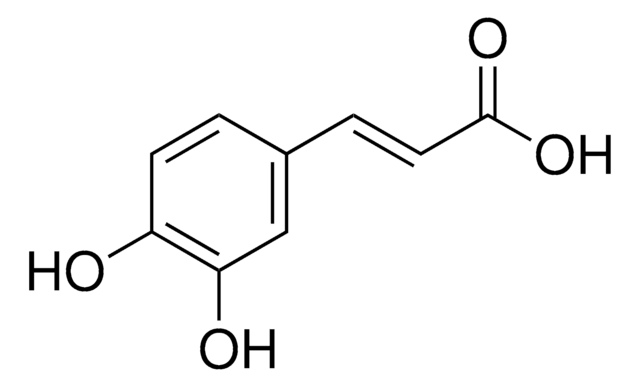
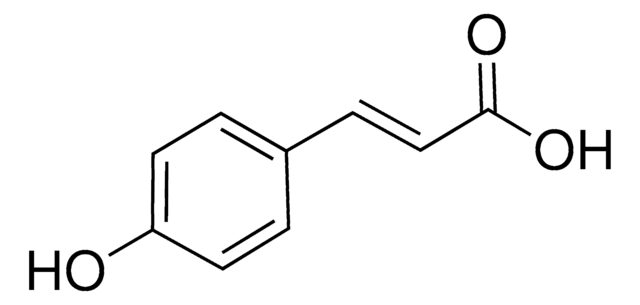
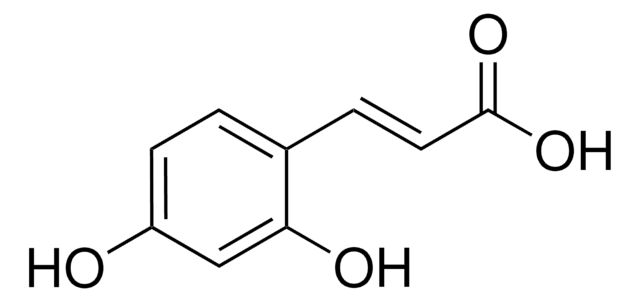
Caffeic acid
matrix substance for MALDI-MS, ≥99.0% (HPLC)
Synonym(s):
3,4-Dihydroxybenzeneacrylic acid, 3,4-Dihydroxycinnamic acid, 3-(3,4-Dihydroxyphenyl)-2-propenoic acid
About This Item
Recommended Products
grade
matrix substance for MALDI-MS
Quality Level
Assay
≥99.0% (HPLC)
form
powder
analyte chemical class(es)
peptides, proteins
technique(s)
MALDI-MS: suitable
color
slightly beige
mp
211-213 °C (dec.) (lit.)
cation traces
Ba: ≤5 mg/kg
Ca: ≤20 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤20 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤20 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
suitability
in accordance for UV test
SMILES string
OC(=O)\C=C\c1ccc(O)c(O)c1
InChI
1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+
InChI key
QAIPRVGONGVQAS-DUXPYHPUSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service