56781
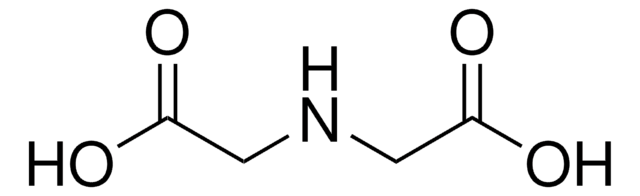
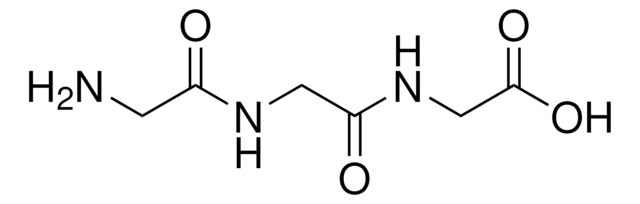
Iminodiacetic acid
purum, ≥98.0% (T)
Synonym(s):
IDA
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
HN(CH2COOH)2
CAS Number:
Molecular Weight:
133.10
Beilstein:
878499
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (T)
form
solid
mp
243 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNCC(O)=O
InChI
1S/C4H7NO4/c6-3(7)1-5-2-4(8)9/h5H,1-2H2,(H,6,7)(H,8,9)
InChI key
NBZBKCUXIYYUSX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Iminodiacetic acid (IDA) can be used as a tridentate ligand to chelate metal ions. Some of the common applications of IDA include:
- Synthesis of N-methyliminodiacetic acid (MIDA), which is an essential precursor for the synthesis of highly versatile MIDA boronates for cross coupling reactions.
- Synthesis of metal complexes of iminodiacetic acid with a radionuclide, especially 99mTc are as radiotracers for cholescintigraphy scans and for radiopharmaceutical applications.
- Synthesis of lanthanide hybrid frameworks via self-assembly of lanthanide ions (Ln3+) with iminodiacetic acid under hydrothermal conditions.
- Preparation of a thermosensitive macroporous protein-imprinted hydrogel for the recognition of protein by metal coordinate interaction.
- Synthesis of IDA functionalized styrene-divinyl benzene co-polymeric beads for the removal of heavy metal ions like Cd(II), Cr(VI), Ni(II) and Pb(II) from their aqueous solutions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Influence of the Denticity of Ligand Systems on the in vitro and in vivo Behavior of 99mTc (I)-Tricarbonyl Complexes: A Hint for the Future Functionalization of Biomolecules.
Schibli R.
Bioconjugate Chemistry, 11(3), 345-351 (2000)
Serendipity in technetium-99m dimethyl iminodiacetic acid cholescintigraphy: diagnosis of nonbiliary disorders in suspected acute cholecystitis.
Weissmann HS.
Radiology, 135(2), 449-454 (1980)
Der chelateffekt.
Schwarzenbach VG.
Helvetica Chimica Acta, 35(7), 2344-2359 (1952)
Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr (VI), Cd (II), Ni (II) and Pb (II) from their aqueous solutions.
Misra RK.
Journal of Hazardous Materials, 185(2-3), 1508-1512 (2011)
General method for synthesis of 2-heterocyclic N-methyliminodiacetic acid boronates.
Dick GR.
Organic Letters, 12(10), 2314-2317 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service