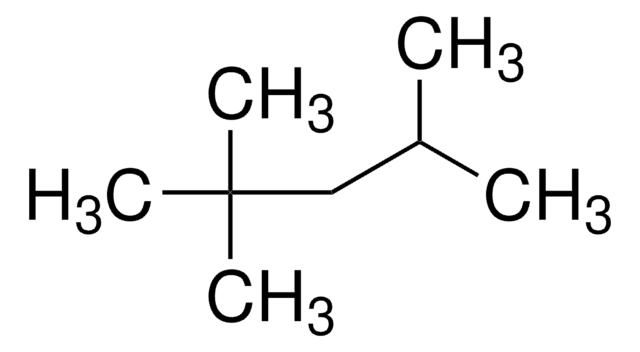
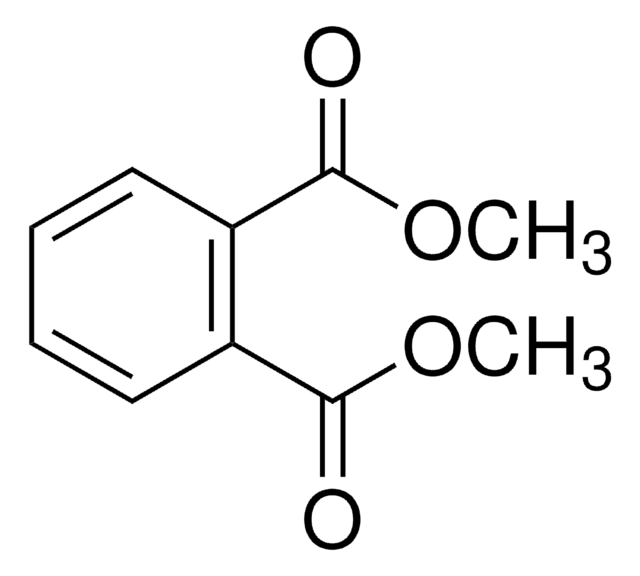
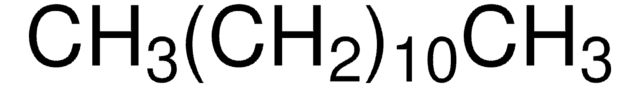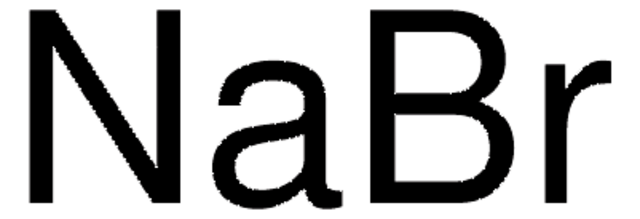36232
Density Standard 1251 kg/m3
H&D Fitzgerald Ltd. Quality
Synonym(s):
Sodium bromide solution
About This Item
Recommended Products
grade
H&D Fitzgerald Ltd. Quality
analytical standard
Quality Level
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
H&D Fitzgerald Ltd.
application(s)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
industrial qc
personal care
petroleum
pharmaceutical (small molecule)
format
mixture
SMILES string
[Na+].[Br-]
InChI
1S/BrH.Na/h1H;/q;+1/p-1
InChI key
JHJLBTNAGRQEKS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Features and Benefits
- Manufactured and verified in an ISO 17025 accredited laboratory, as per UKAS
- Carries an associated uncertainty of +/- 0.010 Kg/m3
- Provided in a sealed glass ampoule of 10 mL to assure safe delivery
- Accompanied with a certificate of analysis (CoA) having information on the expiry date for an effective audit
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service