All Photos(1)
About This Item
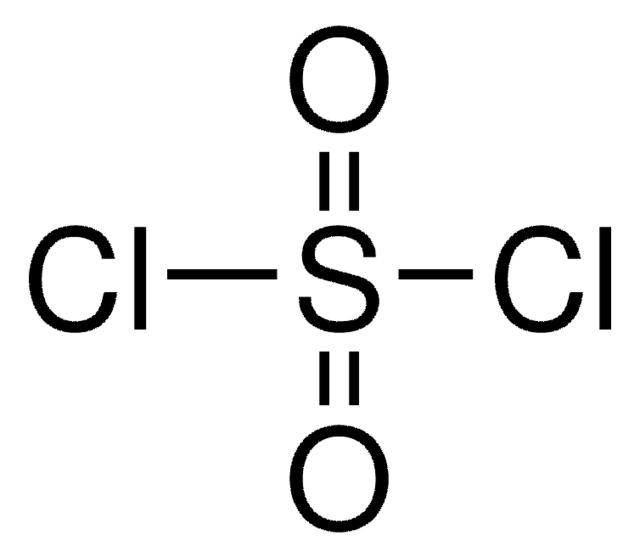
Linear Formula:
SO2Cl2
CAS Number:
Molecular Weight:
134.97
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
4.7 (vs air)
Quality Level
vapor pressure
100 mmHg ( 17.8 °C)
105 mmHg ( 20 °C)
Assay
97%
form
liquid
refractive index
n20/D 1.443 (lit.)
bp
68-70 °C (lit.)
mp
−54 °C (lit.)
density
1.665 g/mL at 20 °C (lit.)
SMILES string
ClS(Cl)(=O)=O
InChI
1S/Cl2O2S/c1-5(2,3)4
InChI key
YBBRCQOCSYXUOC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sulfuryl chloride is a sulfur containing reagent. It is widely used for chlorination of various compounds, as it dissociates into sulfur dioxide and chlorine during reaction. Thus, it acts as a source of molecular chlorine for various aromatic chlorination reactions. Its chlorination reaction with dimethyl sulfide has been studied. Sulfuryl chloride may be used as an efficient reagent for the p-chlorination of phenols.
Application
Sulfuryl chloride (SO2Cl2) may be used in the following studies:
Sulfuryl chloride may be used as an efficient reagent for the p-chlorination of phenols.
- Synthesis of α-chloroketones.
- Regioselective (ortho-selective) chlorination of phenols.
- Conversion of monocyclic allylic cis-1,2-diols to the corresponding trans-1,2-chlorohydrins.
- Preparation of β-chlorotetrahydrofuran derivatives.
Sulfuryl chloride may be used as an efficient reagent for the p-chlorination of phenols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Eye Dam. 1 - Skin Corr. 1C - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Derek R Boyd et al.
Organic & biomolecular chemistry, 12(13), 2128-2136 (2014-02-27)
Monocyclic allylic cis-1,2-diols reacted with sulfuryl chloride at 0 °C in a regio- and stereo-selective manner to give 2-chloro-1-sulfochloridates, which were hydrolysed to yield the corresponding trans-1,2-chlorohydrins. At -78 °C, with very slow addition of sulfuryl chloride, cyclic sulfates were
Chlorinations with sulfuryl chloride. I. The peroxide-catalyzed chlorination of hydrocarbons.
Kharasch MS and Brown HC.
Journal of the American Chemical Society, 61(8), 2142-2150 (1939)
The chlorination of active hydrogen compounds with sulfuryl chloride. I. Ketones.
Wyman DP and Kaufman PR.
The Journal of Organic Chemistry, 29(7), 1956-1960 (1964)
Chlorination of Dimethyl Sulfide and Some of Its Derivatives with Sulfuryl Chloride and Thionyl Chloride1.
Truce WE, et al.
Journal of the American Chemical Society, 74(14), 3594-3599 (1952)
Highly regioselective ortho-chlorination of phenol with sulfuryl chloride in the presence of amines.
Gnaim JM and Sheldon RA.
Tetrahedron Letters, 36(22), 3893-3896 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service