General description
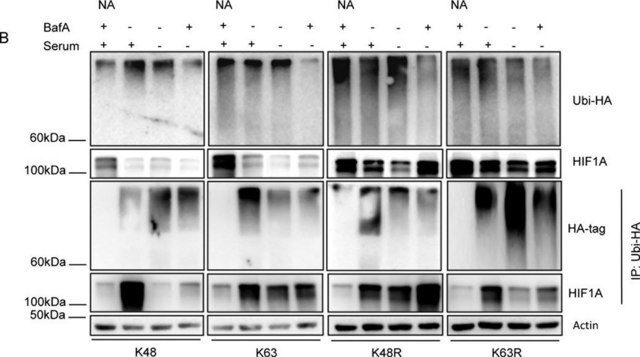
Ubiqutin (Ub) is initially produced as a 229-amino acid Polyubiquitin-B (UniProt P0CG47) precursor protein that is encoded by the UBB gene (Gene ID: 7314) or a 685-amino acid Polyubiquitin-C precursor protein (UniProt P0CG48) encoded by the UBC gene (Gene ID: 7316) in human. Post-translational cleavages yield multiple copies of identical 76-amino acid ubiquitin (also known as high mobility group nonhistone protein HMG-20) molecules. Ubiquitination is one of the most common post-translational modifications (PTMs) of cellular proteins, where Ub is linked covalently via its carboxyl terminus (Gly76) to usually lysine residues in target proteins. In a given target, lysine residue can be linked to one single Ub molecule (monoubiquitylated) or to a chain of Ub molecules (polyubiquitylated). In a polyUb chain, Ub molecules can be linked through one of the seven Lys residues (K6, K11, K27, K29, K33, K48, and K63) or through the Ub N-terminus Met1 residue (which generates linear chains). Lys-C is an endopeptidase that cleaves proteins on the C-terminal side of unmodified lysine residues, but not Ub-modified Lysine residues, generating peptide fragments with a C-terminus Lysine and internal Lysine residue(s) linked to a Ub C-terminal remnant (as a result of Lys-C cleavage between Ub K63 and E64). Antibodies that specifically target this Ub C-terminal remnant (i.e., E64 to Gly76 or E48 to Gly76 if K63 is ubiquitinated in a polyUb chain) are compatible with the patented UbiSite® methodology for Lysine ubiquitination site determination developed by the laboratory of Dr. Blagoy Blagoev at University of Southern Denmark. Such antibodies can isolate Lys-C digested fragments with Ub modification site(s), followed by further trypsin digestion to leave only Gly75-Gly76 covalently linked to the modified Lysine on the isolated peptide fragments prior to Mass spectroscopy (MS) analysis to determine the Lysine modification site(s). They have also use UbiSite® technology to identify serine and threonine residues undergoing HOIL-1 catalyzed ubiquitylation. Threonine residues 12, 14, 22 and serine 20 of ubiquitin are reported to form ester bonds with the C-terminal carboxylate of another ubiquitin molecule thereby increasing the number of ubiquitin linkage types. Serine 175 of IRAK4 and serine 136, 168 and threonine 168 of IRAK2 have been recognized as HOIL-1 catalyzed ubiquitination sites. (Ref.: McCrory, EH., et al. (2022). Biochem. J. 479(23); 2419-2431; Akimov, V., et al. (2018). Nat. Struct. Mol. Biol. 25(7):631-640).
Specificity
Broad species reactivity expected based on 100% sequence homology.
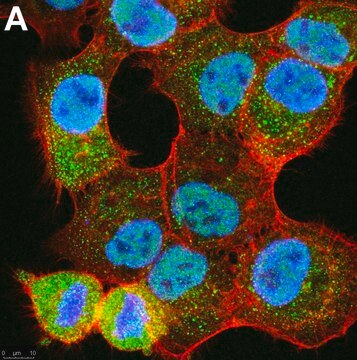
Clone 2G7B8 detects monoubiquitinated (MonoUb) and polyubiquitinated (polyUb) proteins, as well as free ubiquitin (Ub) molecule by targeting Ub sequence C-terminal to Lys63 (UbiSite tag). Clone 2G7B8 can be used to detect (e.g. by Dot blot) and isolate (i.e. by immunoprecipitation) peptides derived from Ub-modified proteins after endoproteinase Lys-C digestion. Lys-C cleaves Ub and Ub-modified proteins after unmodified Lysine residues, but not Ub-modified Lysine residues, generating peptide fragments with their modified lysine residues linked to Ub C-terminal remnant (UbiSite tag) that cannot be recognized by other ubiquitin antibodies whose epitopes lie N-terminally to Lys63. The isolated peptide fragments can then be subjected to Mass spectroscopy (MS) analysis to allow identification of ubiquitin-modification sites.
Immunogen
Epitope: Near C-terminus.
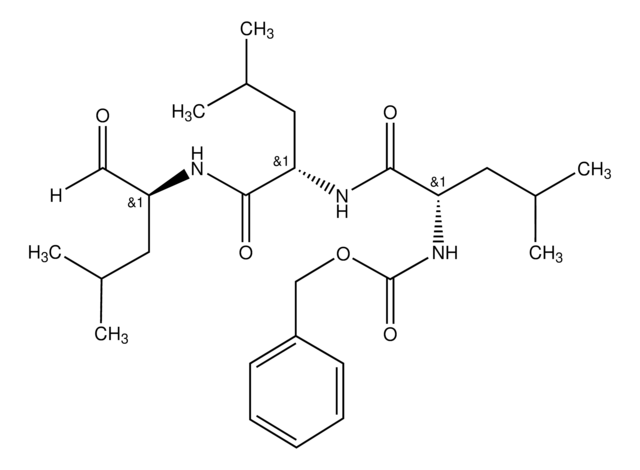
KLH-conjugatedlinear peptide corresponding to 13 amino acids from the C-terminal region ofhuman ubiquitin.
Application
Anti-Ubiquitin(pan)(also known as Anti-Ubiquitin, UbiSite, clone 2G7B8) is a mouse monoclonalantibody that detects Ubiquitin and is used in Western Blotting and Massspectrometry.
Research Category
Signaling
Research Sub Category
Ubiquitin & Ubiquitin Metabolism
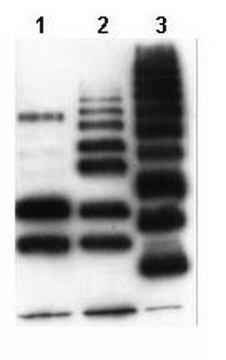
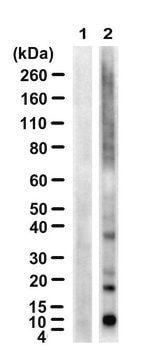
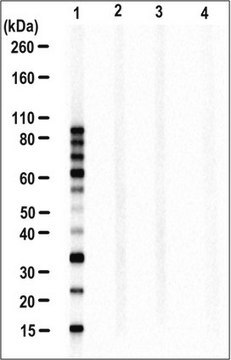
Western Blotting Analysis: 1.0 µg/mL from a representative lot detected the accummulation of ubiquitinated proteins in HEp-2 human epithelial cells following proteasome inhibitor MG-132 (Cat. No. 474790) treatment (Courtesy of Dr. Blagoy Blagoev, University of Southern Denmark).
Quality
Evaluated by Western Blotting in HEp-2 human epithelial cell lysate.
Western Blotting Analysis: 2.0 µg/mL of this antibody detected ubiquitinated proteins in 10 µg of HEp-2 human epithelial cell lysate.
Target description
Variable depending on the size of the ubiquitinated protein(s)/peptide(s) and the degrees of ubiquitination.
Physical form
Format: Purified
Protein G Purified
Purified mouse monoclonal IgG1κ antibody in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide.
Storage and Stability
Stable for 1 year at 2-8°C from date of receipt.
Other Notes
Concentration: Please refer to lot specific datasheet.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.