General description
Major histocompatibility complexes (MHCs), also called human leukocyte antigens (HLAs) in human, are cell surface molecules that play a major part of the immune system in all vertebrates by determining histocompatibility. The main function of MHCs is to bind and present peptide fragments derived from pathogens on the surface of antigen presenting cells. MHCs are divided into three classes, class I MHCs are heterodimers composed of a transmembrane alpha chain and a common extracellular beta subunit. The alpha chain contains three domains (alpha1, alpha2, and alpha3), with the alpha1 domain mediating interaction with the beta2 subunit. The alpha1 and alpha2 domains form the antigen-binding groove. Class II MHCs are heterodimers of two single transmembrane chains, alpha and beta, each contains two domains, with the N-terminal alpha1 and beta1 domain from each chain forming the antigen-binding groove. Class III molecules include several secreted proteins with immune functions, including components of the complement system, cytokines, and heat shock proteins. Each human cell expresses six MHC class I alleles (one HLA-A, -B, and -C allele from each parent). Classical MHCs present antigens to the TCRs of CD8+ T lymphocytes. Nonclassical molecules (class IB MHC) exhibit limited polymorphism, expression patterns, and presented antigens. This group is subdivided into a group encoded within MHC loci (e.g. HLA-E, -F, -G) and those not (e.g. ULBPs, Rae1, and H60).
The previously assigned protein identifier P30443 has been merged into P04439. Full details can be found on the UniProt database.
Specificity
Clone W6/32 is reported to bind murine, rat, rabbit and guinea pig HLA class I heavy chains only when they are associated with bovine β2-microglobulin (β2-m), but not with their respective autologous β2-m (Kahn-Perles, B., et al. (1987). J. Immunol. 138(7).2190-2196).
Clone W6/32 targets HLA classe I heavy chains (HLA-A, -B, -C, -E, and possibly -F) in a light chain-dependent manner, although clone W6/32 does not exhibit affinity toward isolated light chain (β2-microglobulin or β2-m). A tight interaction between β2-m residue 89 and some residues within the heavy chain second domain might be responsible for the formation of the W6/32 antigenic determinant which, however, is only expressed (or exposed) when β2-m cannot covalently bind to the heavy chain Cys121 (Kahn-Perles, B., et al. (1987). J. Immunol. 138(7).2190-2196). Cell surface MHC I molecules reconstituted with allele-specific peptides and recombinant human β2-m that contains a methionine at the N-terminus is not recognized by W6/32 (Shields, M.J., and Ribaudo, R.K. (1998). Tissue Antigens. 51(5):567-570). Clone W6/32 is reported to bind murine, rat, rabbit and guinea pig HLA class I heavy chains only when they are associated with bovine β2-m, but not with their respective autologous β2-m (Kahn-Perles, B., et al. (1987). J. Immunol. 138(7).2190-2196).
Immunogen
Membrane from human tonsil lymphocyte preparations (Barnstable, C.J., et al. (1978). Cell. 14(1):9-20).
Application
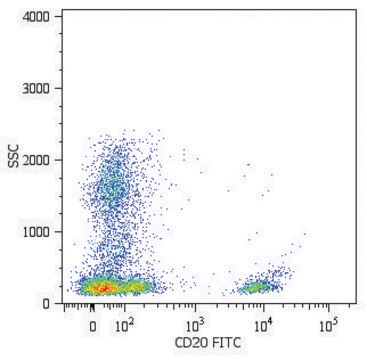
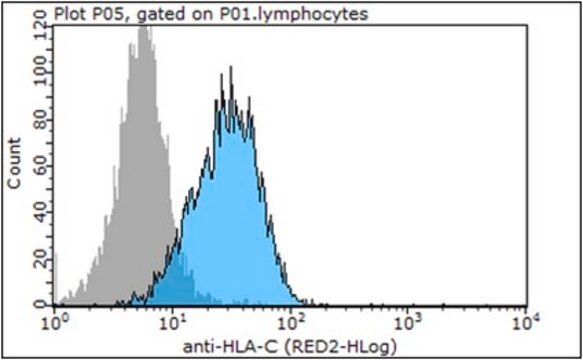
Anti-HLA Class I Antigens Antibody, clone W6/32, Cat. No. MABN1783, targets HLA class I antigens and has been tested in Affinity Binding, Flow Cytometry, Immunoaffinity Purification, Immunohistochemistry, Immunoprecipitation, and Neutralization applications.
Quality
Evaluated by Immunohistochemistry in human tonsil tissue.
Immunohistochemistry Analysis: A 1:1,000 dilutioin of this antibody detected HLA class I antigens in acetone-fixed frozen human tonsil tissue sections.
Target description
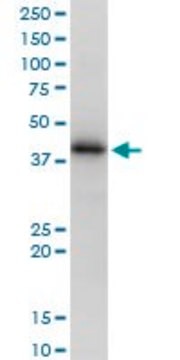
38.32 kDa (HLA-A; Uniprot P30443), 37.90 kDa (HLA-B; Uniprot Q96DW9), 38.14 kDa (HLA-C; Uniprot O19617), 37.92 kDa (HLA-E; Uniprot P13747) calculated based on the mature polymorphic forms specified by the UniProt IDs. ~43 kDa reported (Parham, P., et al. (1979). J. Immunol. 123(1): 342-349; Barnstable, C.J., et al. (1978). Cell. 14(1):9-20).
Physical form
Format: Purified
Purified mouse monoclonal antibody IgG2a in PBS without preservatives.
Other Notes
Concentration: Please refer to lot specific datasheet.
Legal Information
GenBank is a registered trademark of United States Department of Health and Human Services