Recommended Products
biological source
human
Quality Level
Assay
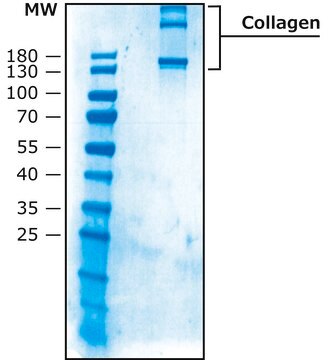
>90% (collagen type I, SDS-PAGE)
form
liquid
manufacturer/tradename
Chemicon®
concentration
1 mg/mL
technique(s)
cell culture | mammalian: suitable
impurities
<1% collagen type II,IV-VI & non-collagen proteins.
<10% collagen type III
input
sample type pancreatic stem cell(s)
sample type mesenchymal stem cell(s)
sample type epithelial cells
sample type induced pluripotent stem cell(s)
sample type neural stem cell(s)
sample type: human embryonic stem cell(s)
sample type hematopoietic stem cell(s)
solubility
water: soluble at 20 °C
NCBI accession no.
UniProt accession no.
Binding Specificity
Peptide Source: Elastin
Peptide Source: Fibronectin
shipped in
dry ice
storage temp.
−20°C
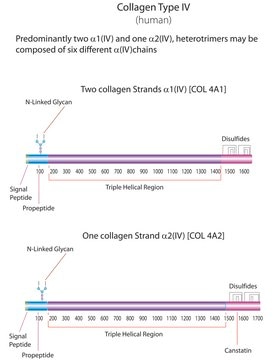
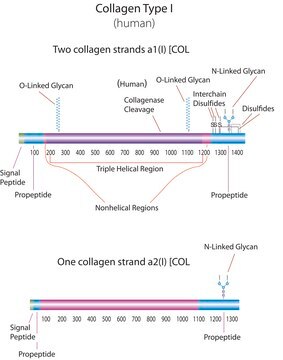
Gene Information
human ... COL1A1(1277)
Related Categories
General description
Application
- as a control in the 2B4 nuclear factor of activated T-cells (NFAT)–GFP reporter cell assay, where its interaction with reporter cells can be evaluated for nuclear factor of activated T-cells (NFAT) promoter-driven GFP expression
- for coating glass slides in dynamic binding assays to create a substrate for the specific binding and study of platelets and conjugates in flow channels
- as a protein standard in histological analysis of lung tissue samples, providing a reference for the composition and characterization of extracellular matrix (ECM) components
Biochem/physiol Actions
Physical form
Analysis Note
Legal Information
Disclaimer
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Extracellular matrix proteins such as laminin, collagen, and fibronectin can be used as cell attachment substrates in cell culture.
Related Content
This page covers the ECM coating protocols developed for four types of ECMs on Millicell®-CM inserts, Collagen Type 1, Fibronectin, Laminin, and Matrigel.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service