341006
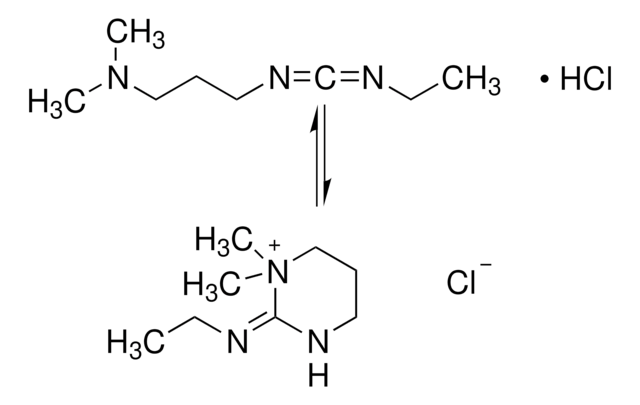
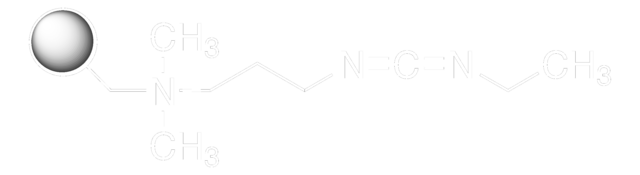
EDAC, Hydrochloride
EDAC HCl is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis.
Synonym(s):
EDAC, Hydrochloride, EDCI, 1-Ethyl-3-(3ʹ-dimethylaminopropyl)carbodiimide, HCl
About This Item
Recommended Products
Quality Level
Assay
≥98% (titration)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated
color
white
solubility
aqueous buffer: 2-5 mg/mL
water: 2-5 mg/mL
shipped in
ambient
storage temp.
−20°C
InChI
1S/C8H17N3.ClH/c1-4-11(8-9)7-5-6-10(2)3;/h4-7H2,1-3H3;1H
InChI key
FDXPUDRRFDHONO-UHFFFAOYSA-N
General description
Packaging
Warning
Preparation Note
Reconstitution
Other Notes
Richardson, A., et al. 1992. Biochem. Pharmacol. 43, 1415.
Taniuchi, M., et al. 1986. Proc. Natl. Acad. Sci. USA83, 1950.
Chase, J.W., et al. 1983. Proc. Natl. Acad. Sci. USA80, 5480.
Williams, A., et al. 1981. J. Am. Chem. Soc.103, 7090.
Yamada, H., et al. 1981. Biochemistry20, 4836.
Thomas, J.O., et al. 1978. J. Mol. Biol.123, 149.
Ozawa, H. 1970. Biochemistry9, 2158.
Kopple, K.D., et al. 1962. J. Am. Chem. Soc.84, 4457.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
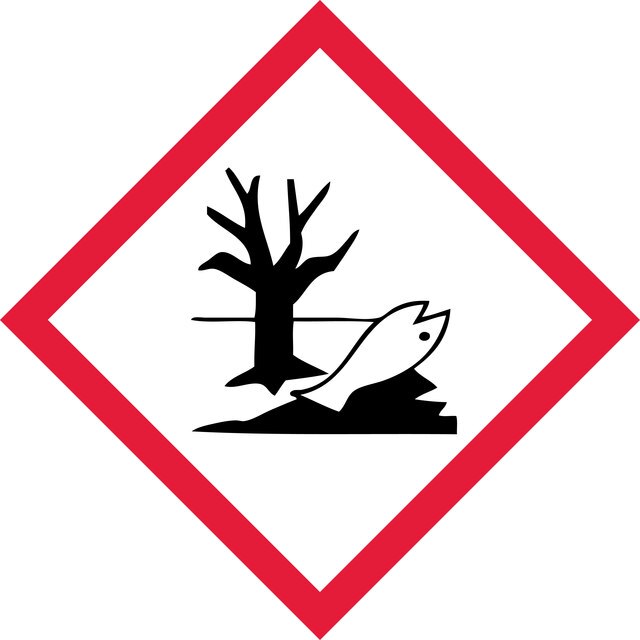
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Target Organs
Stomach,large intestine,lymph node
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service