126654
Albumin, Human Serum, Non-denatured
Synonym(s):
Albumin, Human Serum, Non-denatured
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Assay
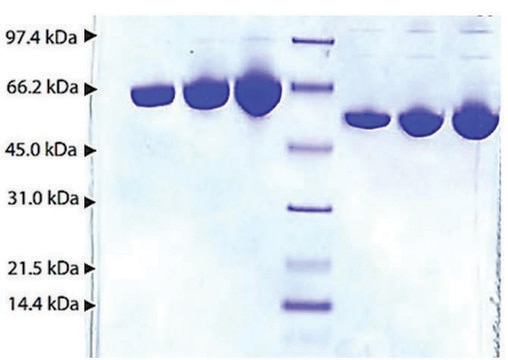
≥95% (SDS-PAGE)
form
lyophilized solid (Salt-free)
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
pI
4.7
solubility
water: 20 mg/mL
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C3F8/c4-1(5,2(6,7)8)3(9,10)11
InChI key
QYSGYZVSCZSLHT-UHFFFAOYSA-N
Related Categories
General description
High purity plasma protein prepared under non-denaturing conditions.
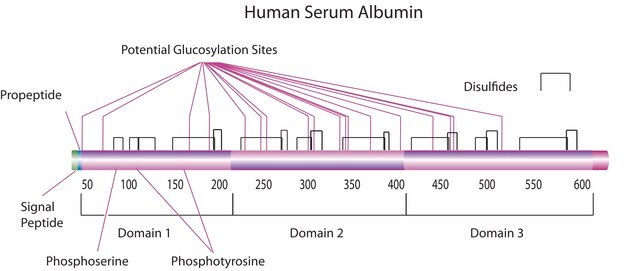
Human serum albumin (HSA) is the most abundant protein present in the plasma. It is a monomeric multi-domain macromolecule comprising three homologous domains, numbered I, II, and III. Each domain consists of subdomains A and B with common structural motifs. The HSA gene is mapped to the human chromosome 4q13.3.
Application
Albumin, human serum, non-denatured has been used:
- to determine the contribution of albumin protein complexes to esterase activity in saliva,
- in tris buffer for monomer degradation studies and in protein adhesion assay
- to study the effect of Cu2+ and Zn2+ ions on human serum albumin interaction with plasma unsaturated fatty acids
Biochem/physiol Actions
Human serum albumin (HSA) functions as a carrier for fatty acids, steroids, and thyroid hormones. It is used as a supplement in cell culture medium to produce vaccines and pharmaceuticals. HSA is a potential biomarker for various diseases, like cancer, rheumatoid arthritis, ischemia, and post-menopausal obesity. It is also used to treat surgical blood loss, trauma, hemorrhage, and cardiopulmonary bypass. HSA is implicated in biological applications such as implantable biomaterials, surgical adhesives and sealants, and bio chromatography.
Warning
Toxicity: Standard Handling (A)
Preparation Note
Prepared from serum that has been shown by certified tests to be negative for HBsAg and for antibodies to HIV and HCV.
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Travis, J., and Pannell, R. 1973. Clin. Chem. Acta49, 49.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kuihua Cai et al.
Dental materials : official publication of the Academy of Dental Materials, 30(8), 848-860 (2014-06-22)
The ester linkages contained within dental resin monomers (such as Bisphenol A-glycidylmethacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA)) are susceptible to hydrolytic degradation by salivary esterases, however very little is known about the specific esterase activities implicated in this process.
Minhtri K Nguyen et al.
American journal of physiology. Renal physiology, 292(5), F1652-F1656 (2007-02-15)
Pseudohyponatremia is a clinical condition characterized by an increased fraction of protein or lipid in plasma, thereby resulting in an artificially low plasma sodium concentration ([Na(+)](p)). Since the automated method of measuring [Na(+)](p) in most laboratories involves the use of
B Grunfeld et al.
Hypertension (Dallas, Tex. : 1979), 15(3), 257-261 (1990-03-01)
Renal functional reserve, microalbuminuria, and plasma atrial natriuretic factor were measured in 21 offspring (9.5 +/- 0.5 years of age, mean +/- SEM) of hypertensive parents and in eight children (10 +/- 0.5 years of age) with no family history
Yang He et al.
Proceedings of the National Academy of Sciences of the United States of America, 108(47), 19078-19083 (2011-11-02)
Human serum albumin (HSA) is widely used in clinical and cell culture applications. Conventional production of HSA from human blood is limited by the availability of blood donation and the high risk of viral transmission from donors. Here, we report
Gabriella Fanali et al.
Molecular aspects of medicine, 33(3), 209-290 (2012-01-11)
Human serum albumin (HSA), the most abundant protein in plasma, is a monomeric multi-domain macromolecule, representing the main determinant of plasma oncotic pressure and the main modulator of fluid distribution between body compartments. HSA displays an extraordinary ligand binding capacity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service