1.10275
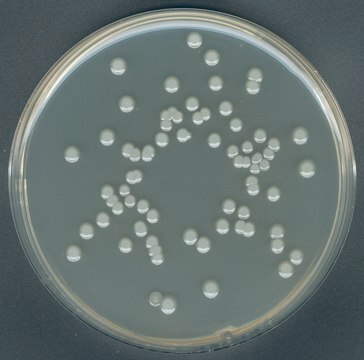
GranuCult® VRBD (Violet Red Bile Dextrose) agar
acc. EP, USP, JP and ISO 21528, For the detection and enumeration of Enterobacteriaceae
Synonym(s):
VRBG Agar. Violet Red Bile Glucose Agar. Crystal-violet neutral-red bile dextrose agar, VRBD-agar (Crystal-violet neutral-red bile dextrose agar) acc. to MOSSEL
About This Item
Recommended Products
Agency
APHA
EP
ISO 21528
JP
USP
Quality Level
sterility
non-sterile
form
granular
manufacturer/tradename
GranuCult® prime
technique(s)
microbiological culture: suitable
pH
7.3 (37 °C, 40 g/L in H2O, after autoclaving)
solubility
39.5 g/L
bulk density
720 kg/m3
application(s)
food and beverages
microbiology
pharmaceutical
storage temp.
15-25°C
suitability
enterobacteriaceae
Related Categories
General description
Application
Footnote
The designations basic, plus, or prime are added to indicate the quality control level, from basic quality control to standard QC plus to prime for full regulatory compliance.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Microbial culture media is available in both powdered and granulated forms. This article compares the characteristics of each culture media format with regards to safety, handling and convenience.
Learn more about our culture media portfolio for microbial examination of non-sterile products, fully compliant with the harmonized Pharmacopoeia.
Protocols
The International Organization for Standardization (ISO) published the revised EN ISO 21528-1 and EN ISO 21528-2 standards in 2017.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service