1.04727
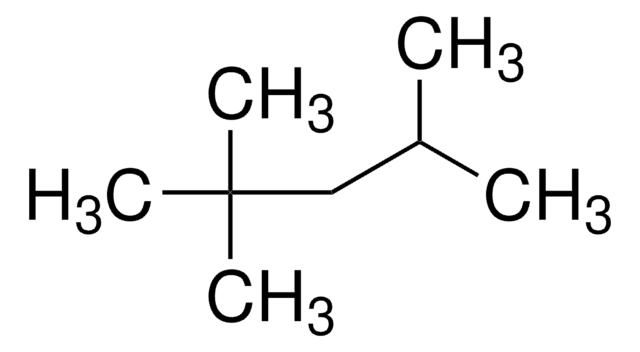
Isooctane
for analysis EMSURE® ACS,Reag. Ph Eur
Synonym(s):
2,2,4-Trimethylpentane, 2,2,4-Trimethylpentane, Isobutyltrimethylmethane, iso-Octane, Isooctane
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Agency
reag. Ph. Eur.
vapor density
3.9 (vs air)
vapor pressure
41 mmHg ( 21 °C)
product line
EMSURE®
Assay
≥99.5% (GC)
form
liquid
autoignition temp.
745 °F
potency
>2500 mg/kg LD50, oral (Rat)
expl. lim.
6 %
impurities
≤0.0003 meq/g Acidity
≤0.005% Sulfur compounds (as S)
≤0.01% Water
evapn. residue
≤0.001%
color
APHA: ≤10
transmittance
250-420 nm, ≥98%
refractive index
n20/D 1.391 (lit.)
pH
7
bp
98-99 °C (lit.)
mp
−107 °C (lit.)
transition temp
flash point -12 °C
density
0.692 g/mL at 25 °C (lit.)
cation traces
Al: ≤0.00005%
B: ≤0.000002%
Ba: ≤0.00001%
Ca: ≤0.00005%
Cd: ≤0.000005%
Co: ≤0.000002%
Cr: ≤0.000002%
Cu: ≤0.000002%
Fe: ≤0.00001%
Mg: ≤0.00001%
Mn: ≤0.000002%
Ni: ≤0.000002%
Pb: ≤0.00001%
Sn: ≤0.00001%
Zn: ≤0.00001%
storage temp.
2-30°C
SMILES string
CC(C)CC(C)(C)C
InChI
1S/C8H18/c1-7(2)6-8(3,4)5/h7H,6H2,1-5H3
InChI key
NHTMVDHEPJAVLT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Behavior of acetyl modified amino acids in reverse micelles: a non-invasive and physiochemical approach.: This study utilizes isooctane in reverse micelles to explore the behavior of acetyl modified amino acids. The findings emphasize isooctane′s role in facilitating non-invasive and physiochemical analyses, highlighting its importance in analytical chemistry and biochemical studies (Mehta et al., 2007).
- Nonionic surfactants: a key to enhance the enzyme activity at cationic reverse micellar interface.: The research investigates the use of isooctane in forming cationic reverse micelles with nonionic surfactants to enhance enzyme activity. Isooctane′s effectiveness in this context underscores its value in biocatalysis and enzymatic studies (Shome et al., 2007).
- Lecithin organogels used as bioactive compounds carriers. A microdomain properties investigation.: This study explores the application of isooctane in lecithin organogels for carrying bioactive compounds. Isooctane′s role in stabilizing the microdomains within the organogels is crucial for their effectiveness in drug delivery systems (Avramiotis et al., 2007).
- Switching electrical conductivity in an AOT-isooctane-water microemulsion through photodimerization of solubilized N-methyl-2-quinolone.: This research demonstrates the use of isooctane in AOT-water microemulsions to switch electrical conductivity via photodimerization. The study highlights isooctane′s utility in developing responsive materials for advanced chemical and physical applications (Bufe and Wolff, 2006).
- Photochromism of crown ethers with incorporated azobenzene moiety.: Isooctane is utilized in studying the photochromism of crown ethers with azobenzene moieties. The findings underscore isooctane′s role in facilitating the investigation of photoresponsive materials, relevant to materials science and photochemistry (Janus and Sworakowski, 2005).
Analysis Note
Identity (IR): conforms
Color: ≤ 10 Hazen
Acidity: ≤ 0.0003 meq/g
Density (d 20 °C/20 °C): 0.691 - 0.696
Refractive index (n 20/D): 1.391 - 1.393
Boiling range (98-100°C): ≥ 95 % (v/v)
Sulfur compounds (as S): ≤ 0.005 %
Readily carbonizable substances: conforms
Transmission (between 250nm and 420nm): ≥ 98 %
Al (Aluminium): ≤ 0.00005 %
B (Boron): ≤ 0.000002 %
Ba (Barium): ≤ 0.00001 %
Ca (Calcium): ≤ 0.00005 %
Cd (Cadmium): ≤ 0.000005 %
Co (Cobalt): ≤ 0.000002 %
Cr (Chromium): ≤ 0.000002 %
Cu (Copper): ≤ 0.000002 %
Fe (Iron): ≤ 0.00001 %
Mg (Magnesium): ≤ 0.00001 %
Mn (Manganese): ≤ 0.000002 %
Ni (Nickel): ≤ 0.000002 %
Pb (Lead): ≤ 0.00001 %
Sn (Tin): ≤ 0.00001 %
Zn (Zinc): ≤ 0.00001 %
Evaporation residue: ≤ 0.001 %
Water: ≤ 0.01 %
Corresponds to ACS, Ph Eur-reagent
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
10.4 °F - closed cup
Flash Point(C)
-12 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service