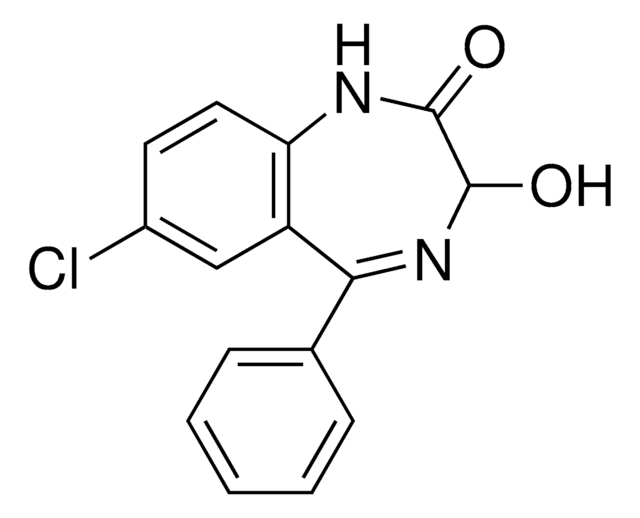
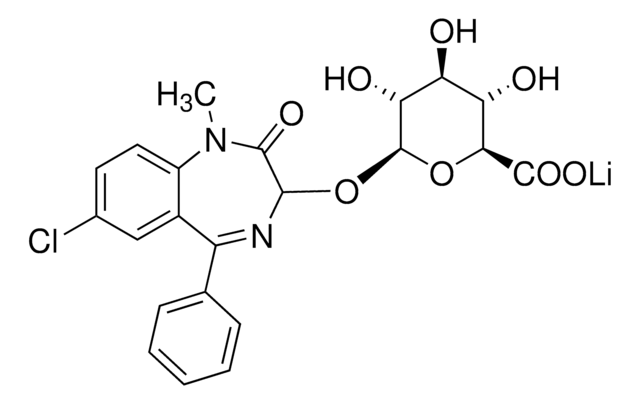
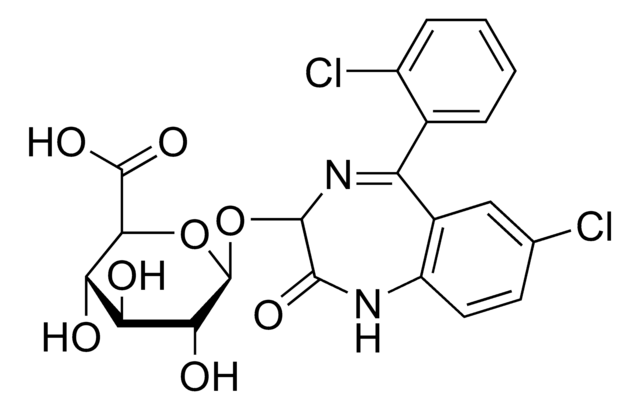
O-023
Oxazepam glucuronide solution
100 μg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland)
concentration
100 μg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
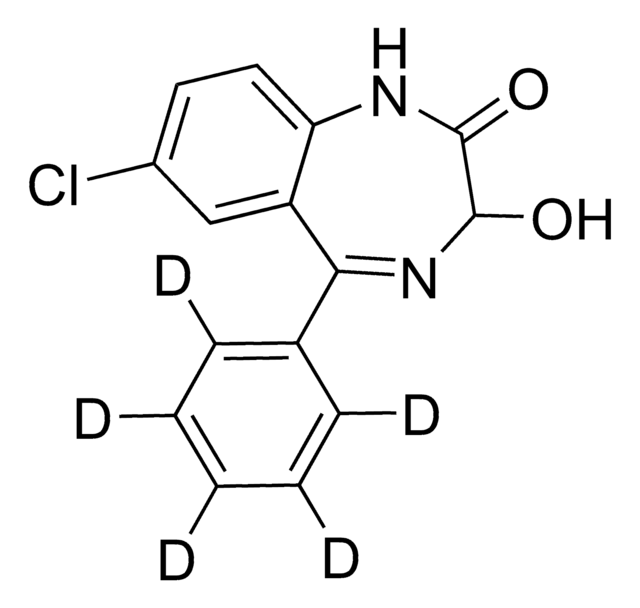
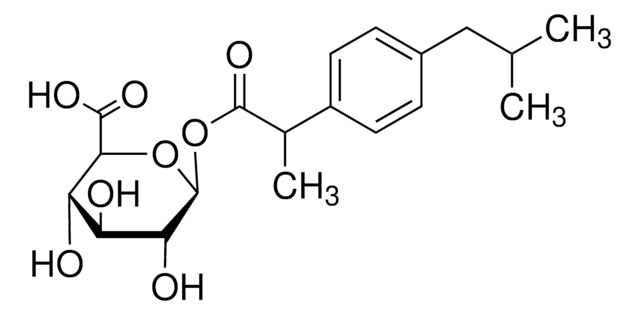
SMILES string
O[C@@H]1[C@@H](O)[C@H](OC2N=C(c3ccccc3)c4cc(Cl)ccc4NC2=O)O[C@@H]([C@H]1O)C(O)=O
InChI
1S/C21H19ClN2O8/c22-10-6-7-12-11(8-10)13(9-4-2-1-3-5-9)24-19(18(28)23-12)32-21-16(27)14(25)15(26)17(31-21)20(29)30/h1-8,14-17,19,21,25-27H,(H,23,28)(H,29,30)/t14-,15-,16+,17-,19?,21-/m0/s1
InChI key
FIKQKGFUBZQEBL-IFBJMGMISA-N
General description
Application
- Advanced Analytical Techniques for Benzodiazepine Metabolites: Oxazepam glucuronide has been targeted in studies using high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). This method allows for the sensitive and automatic determination of diazepam and its metabolites, including oxazepam glucuronide, in human oral fluid, highlighting its utility in forensic and clinical toxicology (Jiang et al., 2016).
- Forensic Applications: Oxazepam glucuronide is directly determined using μElution solid-phase extraction and liquid chromatography-tandem mass spectrometry (LC-MS/MS) in human whole blood. This technique is crucial for forensic science, providing reliable data for legal and medical investigations involving benzodiazepine use (Wang et al., 2013).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service