H-052
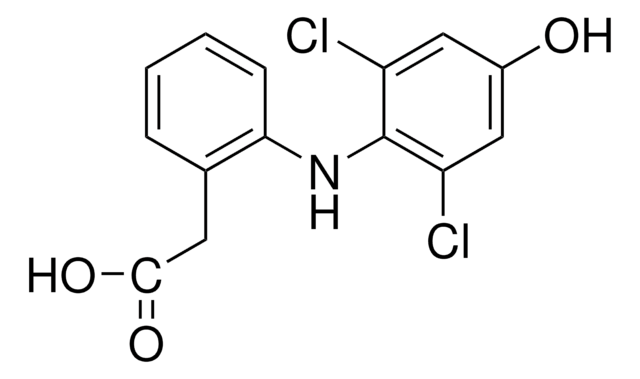
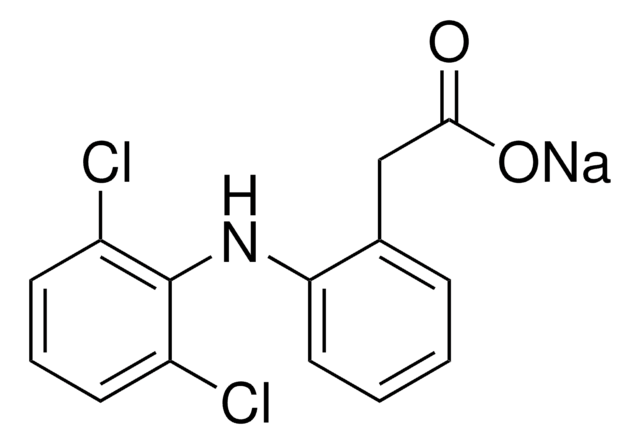
4′-Hydroxydiclofenac solution
100 μg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
100 μg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
OC(=O)Cc1ccccc1Nc2c(Cl)cc(O)cc2Cl
InChI
1S/C14H11Cl2NO3/c15-10-6-9(18)7-11(16)14(10)17-12-4-2-1-3-8(12)5-13(19)20/h1-4,6-7,17-18H,5H2,(H,19,20)
InChI key
KGVXVPRLBMWZLG-UHFFFAOYSA-N
General description
Application
- Metabolism of non-steroidal anti-inflammatory drugs (NSAIDs): This study examines the biotransformation of 4′-Hydroxydiclofenac by Streptomyces griseolus CYP105A1, emphasizing its relevance in pharmacokinetic research on drug metabolism and enzyme inhibition studies (Yogo et al., 2022).
- A multi-biomarker approach to assess toxicity of diclofenac and 4-OH diclofenac: Investigates the environmental impact of 4′-Hydroxydiclofenac on molluscs, providing insights into the ecological side-effects of pharmaceutical residues and their metabolites, important for researchers studying drug metabolism and environmental safety (Swiacka et al., 2022).
- Cytochrome P450 Expression and Chemical Metabolic Activity before Full Liver Development in Zebrafish: Highlights the role of 4′-Hydroxydiclofenac in early developmental stages of liver metabolism in zebrafish, useful for developmental pharmacokinetics and toxicology studies (Nawaji et al., 2020).
- Different Roles of Human Cytochrome P450 2C9 and 3A Enzymes in Diclofenac 4′- and 5-Hydroxylations: Explores the specific roles of cytochrome P450 enzymes in the metabolism of diclofenac and its major metabolite 4′-Hydroxydiclofenac, offering critical insights for pharmacokinetic research and drug metabolism assays (Miura et al., 2020).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service