All Photos(1)
About This Item
Empirical Formula (Hill Notation):
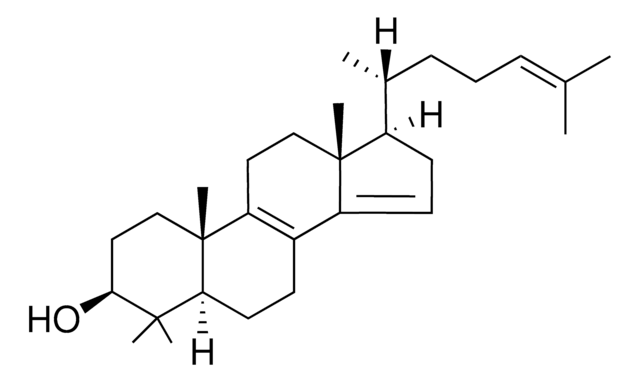
C27H46O
CAS Number:
Molecular Weight:
386.65
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (700118P-1mg)
manufacturer/tradename
Avanti Polar Lipids 700118P
shipped in
dry ice
storage temp.
−20°C
InChI
1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h18-21,23-24,28H,6-17H2,1-5H3/t19-,20+,21+,23-,24+,26+,27-/m1/s1
InChI key
QETLKNDKQOXZRP-XTGBIJOFSA-N
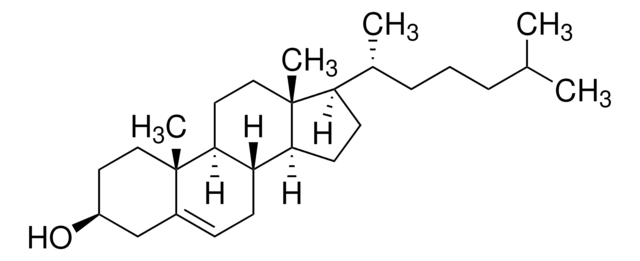
General description
Zymostenol acts as a substrate for Δ8,7-sterol isomerase (EBP). It is a demethylation product of 24,25-dihydrolanosterol (24,25-DHL).
Application
Zymostenol has been used to study its accumulation and its inhibitory actions on Δ8,7-sterol isomerase (EBP).
Packaging
5 mL Amber Glass Screw Cap Vial (700118P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diverse Chemical Scaffolds Enhance Oligodendrocyte Formation by Inhibiting CYP51, TM7SF2, or EBP
Allimuthu D, et al.
Cell Chemical Biology (2019)
Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol
Song BL, et al.
Cell Metabolism, 1(3), 179-189 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service