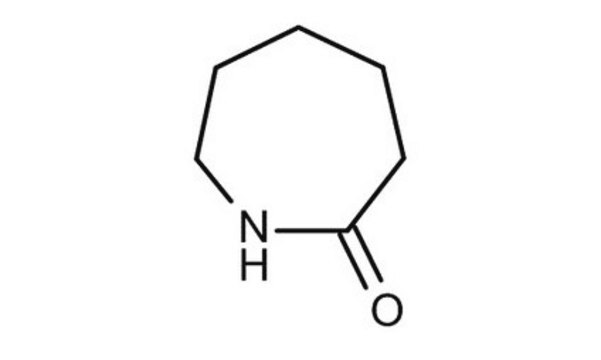
V209
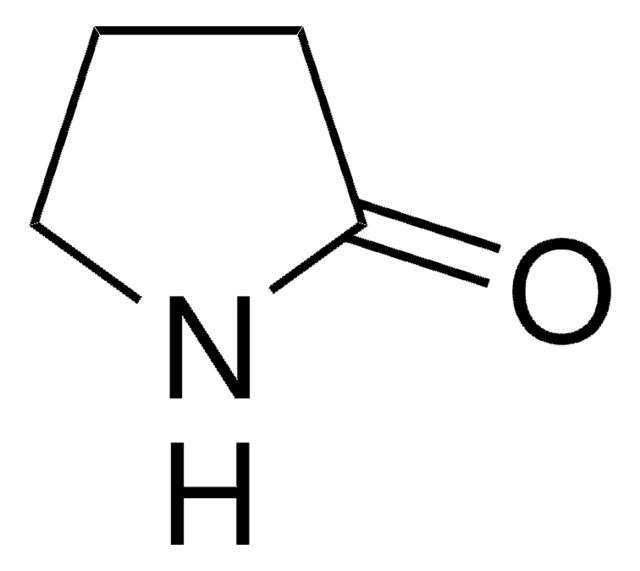
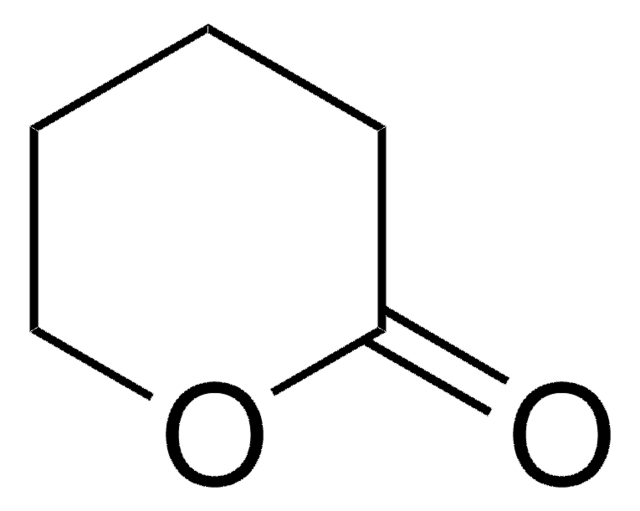
δ-Valerolactam
98%
Synonym(s):
delta-Valerolactam, 2-Piperidone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO
CAS Number:
Molecular Weight:
99.13
Beilstein:
106434
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
256 °C (lit.)
81-82 °C/0.1 mmHg (lit.)
mp
38-40 °C (lit.)
SMILES string
O=C1CCCCN1
InChI
1S/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
InChI key
XUWHAWMETYGRKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ahmed Mahjoub et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(10), 1822-1832 (2011-05-28)
We studied the single-photon ionization of gas-phase δ-valerolactam (piperidin-2-one) and of its dimer using vacuum-ultraviolet (VUV) synchrotron radiation coupled to a velocity map imaging electron/ion coincidence spectrometer. The slow photoelectron spectrum (SPES) of the monomer is dominated by the vibrational
Metabolic engineering of Escherichia coli for the production of four-, five- and six-carbon lactams.
Tong Un Chae et al.
Metabolic engineering, 41, 82-91 (2017-04-10)
Microbial production of chemicals and materials from renewable sources is becoming increasingly important for sustainable chemical industry. Here, we report construction of a new and efficient platform metabolic pathway for the production of four-carbon (butyrolactam), five-carbon (valerolactam) and six-carbon (caprolactam)
Moitrayee Mukherjee et al.
The journal of physical chemistry. A, 116(40), 9888-9896 (2012-09-19)
A comparative analysis for relative stability between normal and tautomeric forms in the excited electronic states of 7-azaindole···δ-valerolactam 1:1 complex and 7-azaindole homodimer has been presented. The tautomeric configuration of the complex is estimated to be ~6 kcal/mol more stable
Asymmetric synthesis of gamma-keto-delta-lactam derivatives: application to the synthesis of a conformationally constrained surrogate of Ala-Ser dipeptide.
S D Koulocheri et al.
The Journal of organic chemistry, 66(23), 7915-7918 (2001-11-10)
S Gordon et al.
Farmaco (Societa chimica italiana : 1989), 52(10), 603-608 (1998-05-15)
The synthesis of a series of 2-amino-4-hydroxy-delta-valerolactam derivatives is described (compounds 4 to 10). These compounds showed a high anthelmintic in vitro activity against the Nippostrongylus brasiliensis model.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service