914789
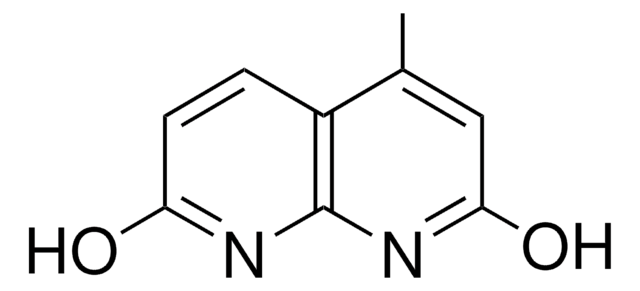
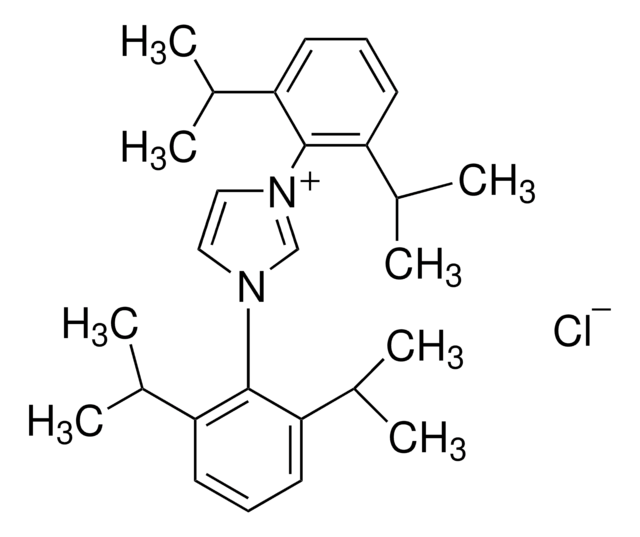
iPr-NDI
≥95%
Synonym(s):
Isopropyl naphthyridine-diimine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C36H44N4
CAS Number:
Molecular Weight:
532.76
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Application
iPr-NDI is a ligand that when mixed with NiBr2, generates an active dinuclear Ni catalyst. The catalyst has been shown to promote a variety of transformations including the cyclopropanation of alkenes and the [4+1] cycloaddition of vinylidenes and dienes.
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Douglas R Hartline et al.
Journal of the American Chemical Society, 139(39), 13672-13675 (2017-09-19)
Single bonds between carbon atoms are inherently challenging to activate using transition metals; however, ring-strain release can provide the necessary thermodynamic driving force to make such processes favorable. In this report, we describe a strain-induced C-C oxidative addition of norbornadiene.
You-Yun Zhou et al.
Angewandte Chemie (International ed. in English), 55(9), 3171-3175 (2016-01-30)
Dinuclear Ni complexes supported by naphthyridine-diimine (NDI) ligands catalyze the reductive cyclopropanation of alkenes with CH2 Cl2 as the methylene source. The use of mild terminal reductants (Zn or Et2 Zn) confers significant functional-group tolerance, and the catalyst accommodates structurally
Ian G Powers et al.
Journal of the American Chemical Society, 140(11), 4110-4118 (2018-03-01)
Azoarenes are valuable chromophores that have been extensively incorporated as photoswitchable elements in molecular machines and biologically active compounds. Here, we report a catalytic nitrene dimerization reaction that provides access to structurally and electronically diverse azoarenes. The reaction utilizes aryl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)