914568
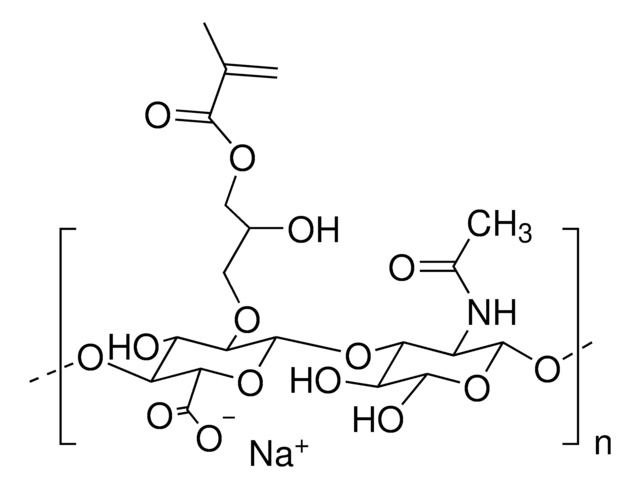
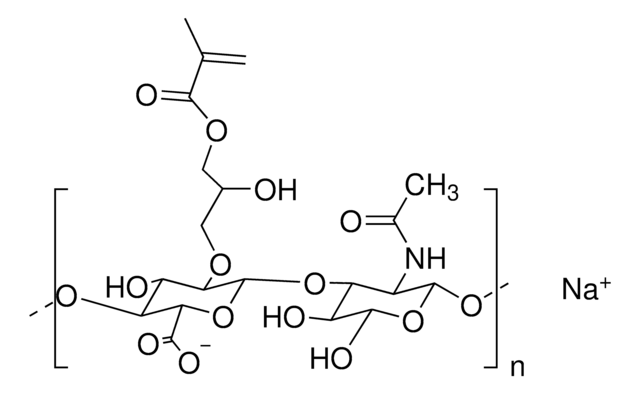
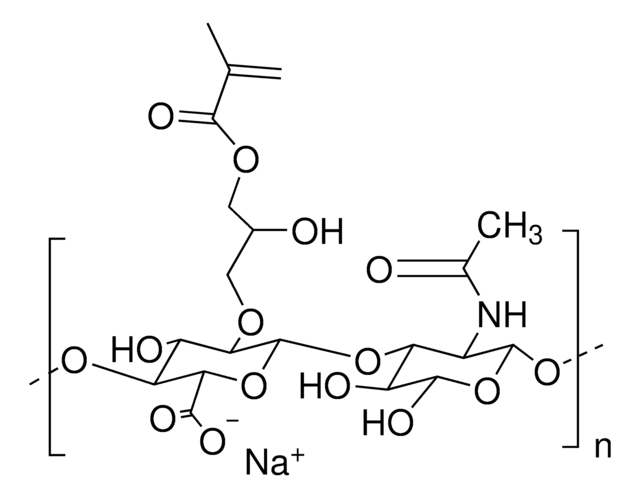
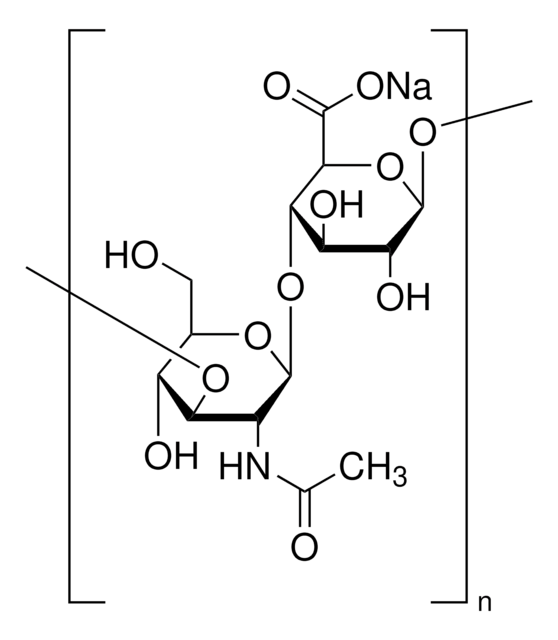
Hyaluronic acid methacrylate
degree of substitution 20-50%, Mw 40-70 kDa
Synonym(s):
HA methacrlamide, HAMA, Hyaluronic acid MA
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(NaC20H28NO15)n
UNSPSC Code:
12352106
Recommended Products
description
NMR: Conforms to structure
Quality Level
form
powder or chunks (or fibers)
mol wt
Mw 40-70 kDa
color
white to off-white
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Hyaluronic acid (HA) is a linear polysaccharide of alternating D-glucuronic acid and N-acetyl-D-glucosamine found primarily in connective tissues. HA based hydrogels are widely used in tissue engineering, 3D bioprinting, and drug deliery applications. The methacrylate functionalized hyaluronic acid is photo-crosslinkable, and can be used to generate crosslinked hydrogels.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cindy Chung et al.
Tissue engineering. Part A, 15(2), 243-254 (2009-02-06)
Mesenchymal stem cells (MSCs) are multipotent progenitor cells whose plasticity and self-renewal capacity have generated significant interest for applications in tissue engineering. The objective of this study was to investigate MSC chondrogenesis in photo-cross-linked hyaluronic acid (HA) hydrogels. Because HA
Judy Yeh et al.
Biomaterials, 27(31), 5391-5398 (2006-07-11)
Encapsulation of mammalian cells within hydrogels has great utility for a variety of applications ranging from tissue engineering to cell-based assays. In this work, we present a technique to encapsulate live cells in three-dimensional (3D) microscale hydrogels (microgels) of controlled
Aleksander Skardal et al.
Tissue engineering. Part A, 16(8), 2675-2685 (2010-04-15)
Bioprinting by the codeposition of cells and biomaterials is constrained by the availability of printable materials. Herein we describe a novel macromonomer, a new two-step photocrosslinking strategy, and the use of a simple rapid prototyping system to print a proof-of-concept
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service