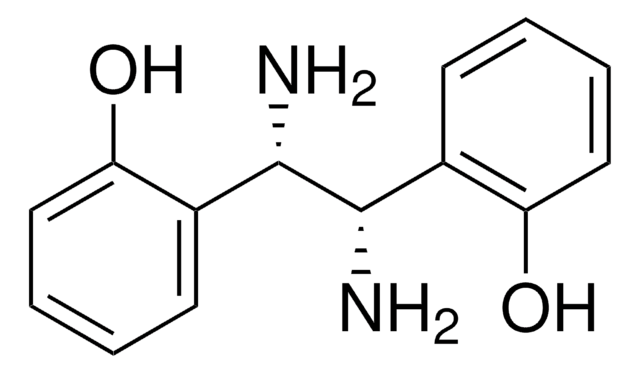
684104
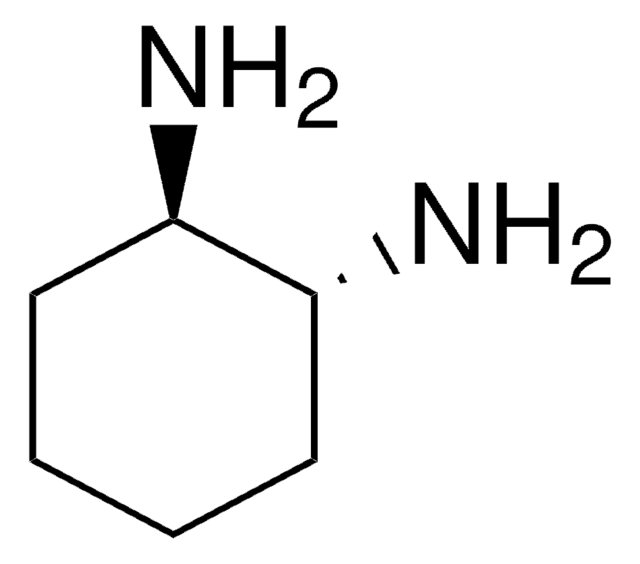
(1S,2S)-1,2-Bis(2,4,6-trimethylphenyl)ethylenediamine dihydrochloride
95%
Synonym(s):
(1S,2S)-1,2-Bis(2,4,6-trimethylphenyl)-1,2-ethanediamine dihydrochloride
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
optical activity
[α]20/D −129°, c = 1 in H2O
mp
>300 °C
SMILES string
Cl.Cl.Cc1cc(C)c([C@H](N)[C@@H](N)c2c(C)cc(C)cc2C)c(C)c1
InChI
1S/C20H28N2.2ClH/c1-11-7-13(3)17(14(4)8-11)19(21)20(22)18-15(5)9-12(2)10-16(18)6;;/h7-10,19-20H,21-22H2,1-6H3;2*1H/t19-,20-;;/m0../s1
InChI key
HSFKFAWYBTVLDG-TULUPMBKSA-N
Related Categories
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Chin group is interested in computational and experimental approaches to understanding stereoselective recognition and catalysis. Their studies in weak forces (H-bonding, electronic and steric effects) has led to a highly efficient method for making limitless varieties of chiral vicinal diamines from the 'mother diamine' that are useful for developing stereoselective organocatalysts or transition metal-based catalysts as well as for developing drugs (Acc Chem Res (2012) p1345). The 'mother diamine' is also useful for making binol, monophos and binap analogs. The Chin group is also interested in using reversible covalent bonds for stereoselective recognition and L to D conversion of natural and non-natural amino acids (EJOC (2012) p229).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)