649317
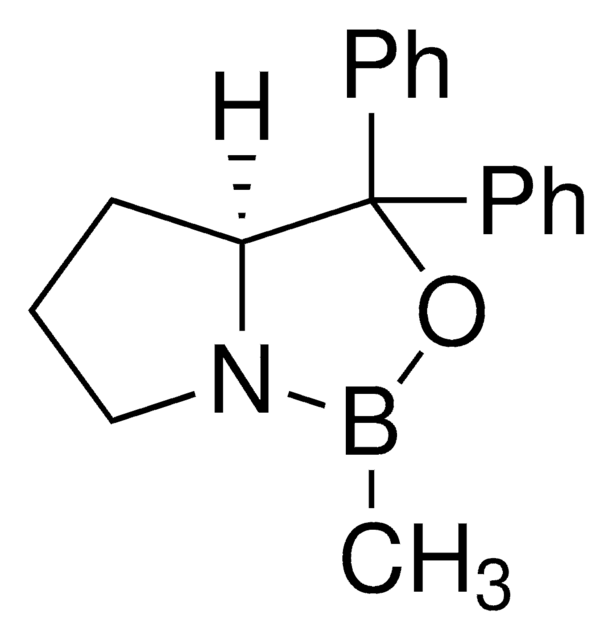
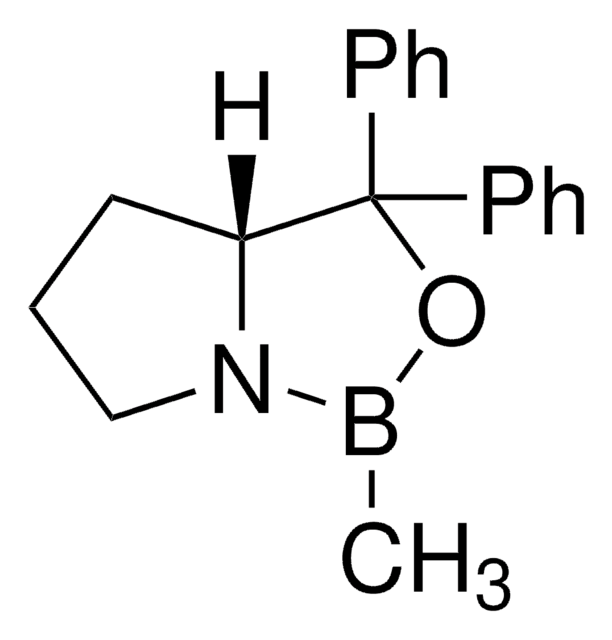
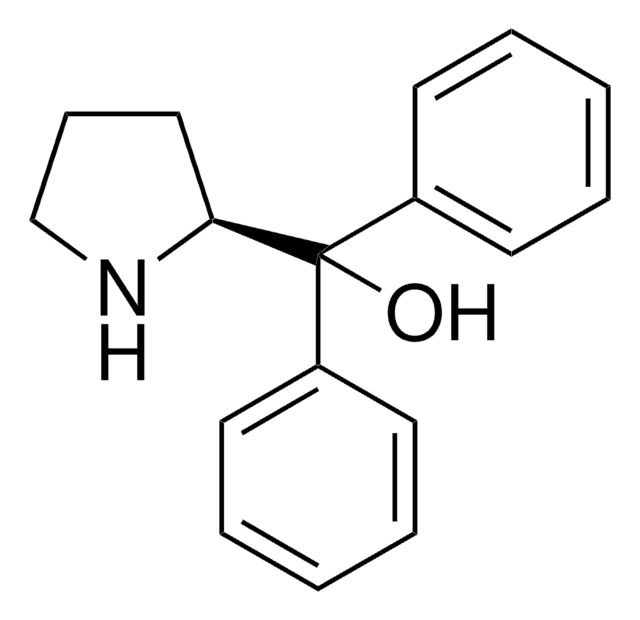
(R)-(+)-2-Methyl-CBS-oxazaborolidine
Synonym(s):
(R)-1-Methyl-3,3-diphenylhexahydropyrrolo[1,2-c][1,3,2]oxazaborole, (R)-3,3-Diphenyl-1-methylpyrrolidino[1,2-c]-1,3,2-oxazaborole, (R)-Me-Corey-Bakshi-Shibata catalyst
About This Item
Recommended Products
Assay
≥90%
Quality Level
form
solid
mp
85-95 °C (lit.)
SMILES string
CB1OC(C2CCCN12)(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m1/s1
InChI key
VMKAFJQFKBASMU-QGZVFWFLSA-N
Related Categories
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
Related Content
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service