All Photos(2)
About This Item
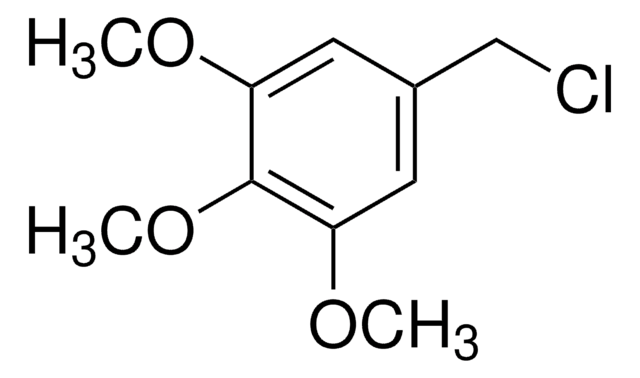
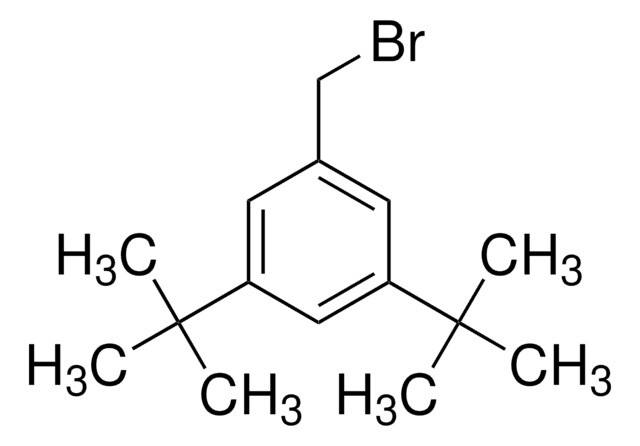
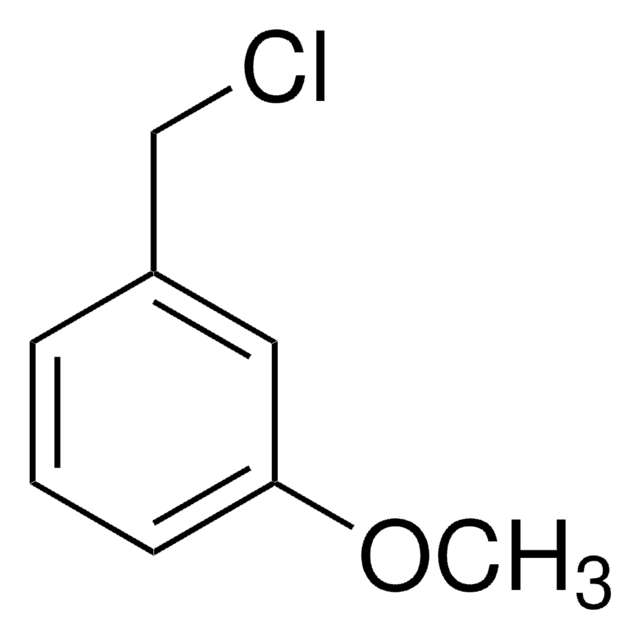
Linear Formula:
(CH3O)2C6H3CH2Br
CAS Number:
Molecular Weight:
231.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
69-70 °C (lit.)
storage temp.
2-8°C
SMILES string
COc1cc(CBr)cc(OC)c1
InChI
1S/C9H11BrO2/c1-11-8-3-7(6-10)4-9(5-8)12-2/h3-5H,6H2,1-2H3
InChI key
BTHIGJGJAPYFSJ-UHFFFAOYSA-N
Related Categories
General description
3,5-Dimethoxybenzyl bromide is an aromatic bromide.
Application
3,5-Dimethoxybenzyl bromide may be used in the synthesis of:
- 3,4,3′,5′-tetramethoxystilbene
- 3,5-dimethoxyphenylacetic acid
- 1-(3,5-dimethoxyphenyl)-4-pentene
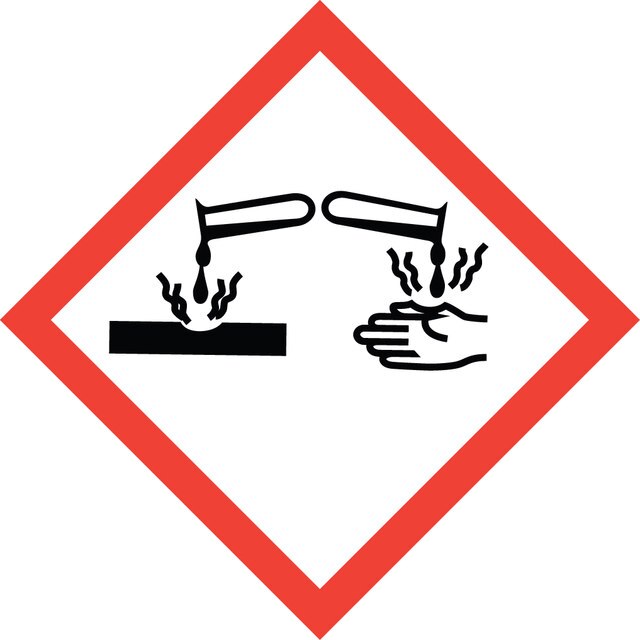
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthese de Δ8 et Δ9-tetrahydrocannabinol deuteries et trities.
Hoellinger H, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 13(3), 401-415 (1977)
An efficient synthesis of 3,5-dimethoxyhomophthalic acid, a key intermediate for synthesis of natural isocoumarins.
Saeed A, et al.
Journal of Heterocyclic Chemistry, 40(3), 519-522 (2003)
Dorota Chełminiak-Dudkiewicz et al.
Journal of photochemistry and photobiology. B, Biology, 181, 1-13 (2018-02-27)
Three magnesium sulfanyl porphyrazines differing in the size of peripheral substituents (3,5-dimethoxybenzylsulfanyl, (3,5-dimethoxybenzyloxy)benzylsulfanyl, 3,5-bis[(3,5-bis[(3,5-dimethoxybenzyloxy)benzyloxy]benzylsulfanyl) were exposed to visible and ultraviolet radiation (UV A + B + C) in order to determine their photochemical properties. The course of photochemical reactions in dimethylformamide solutions and the
Synthesis of pinosylvin and related heartwood stilbenes.
Bachelor FW, et al.
Canadian Journal of Chemistry, 48(10), 1554-1557 (1970)
Synthesis of resveratrol, DMU-212 and analogues through a novel Wittig-type olefination promoted by nickel nanoparticles.
Alonso F, et al.
Tetrahedron Letters, 50(25), 3070-3073 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service