All Photos(1)
About This Item
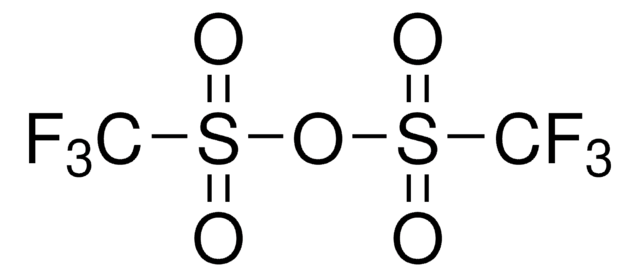
Empirical Formula (Hill Notation):
C7H9F3O3S
CAS Number:
Molecular Weight:
230.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
84-87 °C/4 mmHg (lit.)
density
1.315 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
FC(F)(F)S(=O)(=O)OC1=CCCCC1
InChI
1S/C7H9F3O3S/c8-7(9,10)14(11,12)13-6-4-2-1-3-5-6/h4H,1-3,5H2
InChI key
WVSCRRLWRRANJY-UHFFFAOYSA-N
Related Categories
General description
1-Cyclohexenyl trifluoromethanesulfonate, also known as 1-cyclohexenyl triflate, is a cyclohexenyl sulfonate. Its trifluoromethylation reaction in the presence of different monodentate biaryl phosphine ligands has been investigated. The asymmetric Heck reaction of 1-cyclohexenyl trifluoromethanesulfonate using palladium complexes of phosphine oxazoline ligand has been studied.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
160.0 °F - closed cup
Flash Point(C)
71.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eun Jin Cho et al.
Organic letters, 13(24), 6552-6555 (2011-11-25)
A method for the palladium-catalyzed trifluoromethylation of cyclohexenyl sulfonates has been developed. Various cyclohexenyl triflates and nonaflates underwent trifluoromethylation under mild reaction conditions using a catalyst system composed of Pd(dba)(2) or [(allyl)PdCl](2) and the monodentate biaryl phosphine ligand (t)BuXPhos. The
Proline derived phosphine-oxazoline ligands in the asymmetric Heck reaction.
Gilbertson SR, et al.
Tetrahedron Letters, 42(3), 365-368 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)