456055
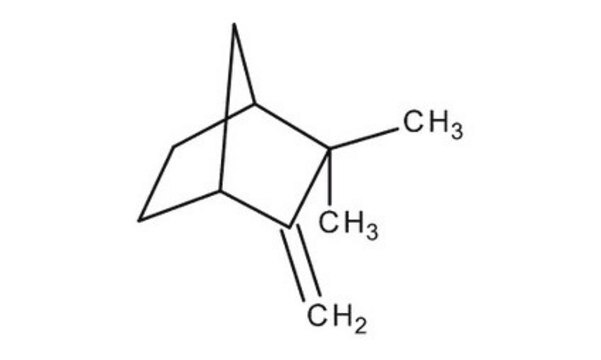
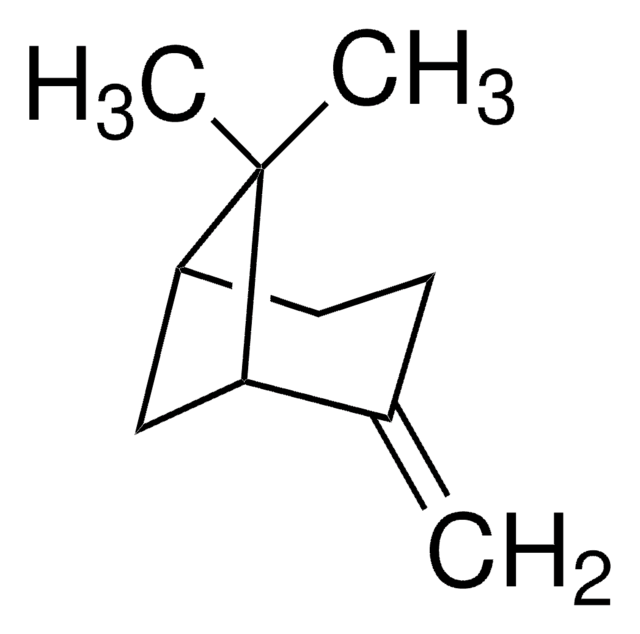
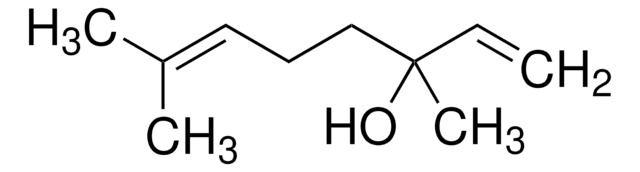
Camphene
95%
Synonym(s):
(±)-Camphene, 2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane, 2,2-Dimethyl-3-methylenenorbornane, 2,2-Dimethyl-3-methylidenebicyclo[2.2.1]heptane, 2-Methylene-3,3-dimethylbicyclo[2.2.1]heptane, 3,3-Dimethyl-2-methylenenorbornane, 3,3-Dimethyl-2-methylenenorcamphane, DL-Camphene
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
bp
159-160 °C (lit.)
mp
48-52 °C (lit.)
density
0.85 g/mL at 25 °C (lit.)
SMILES string
[H][C@]12CC[C@]([H])(C1)C(C)(C)C2=C
InChI
1S/C10H16/c1-7-8-4-5-9(6-8)10(7,2)3/h8-9H,1,4-6H2,2-3H3/t8-,9+/m0/s1
InChI key
CRPUJAZIXJMDBK-DTWKUNHWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Isobornyl carboxylates by silica-supported tungstophosphoric acid-catalyzed liquid-phase esterification of C2-C6 fatty acids.
- Hydroaminated camphene via intermolecular anti-Markovnikov hydroamination reaction with N-hydroxyphthalimide and triethyl phosphite in the presence of dilauroyl peroxide as an initiator.
- Camphene oxide via methyltrioxorhenium-catalyzed epoxidation in the presence of H2O2 as an oxidant and pyrazole as a Lewis base adduct.
- Isobornyl alkyl ethers using alcohols via cation exchange resin-catalyzed alkoxylation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Sol. 1
WGK
WGK 2
Flash Point(F)
DIN 51755 Part 1
Flash Point(C)
DIN 51755 Part 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service