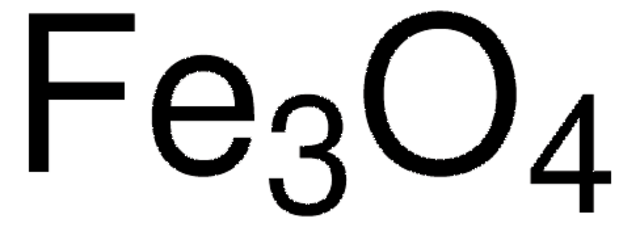310069
Iron(II,III) oxide
powder, <5 μm, 95%
Synonym(s):
Ferrosoferric oxide, Iron oxide black, Magnetite
About This Item
Recommended Products
Quality Level
Assay
95%
form
powder
particle size
<5 μm
mp
1538 °C (lit.)
density
4.8-5.1 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Fe].O=[Fe]O[Fe]=O
InChI
1S/3Fe.4O
InChI key
SZVJSHCCFOBDDC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A starting material to synthesize Ca2Fe2O5 (srebrodolskite) microspheres via a single-stage flame spheroidisation (FS) process.
- A catalyst for reverse water gas shift reactions(RWGS).
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Randal Lee (University of Houston, USA) discusses design considerations for iron oxide magnetic nanospheres and nanocubes used for biosensing, including synthetic procedures, size, and shape. The effects of these variables are discussed for various volumetric-based and surface-based detection schemes.
An article concerning self-propagating reactions induced by mechanical alloying, presented by Sigma-Aldrich.com.
Magnetic materials permeate numerous daily activities in our lives. They are essential components of a diversity of products including hard drives that reliably store information on our computers, decorative magnets that keep the shopping list attached to the refrigerator door, electric bicycles that speed our commute to work, as well as wind turbines for conversion of wind energy to electrical power.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service