258202
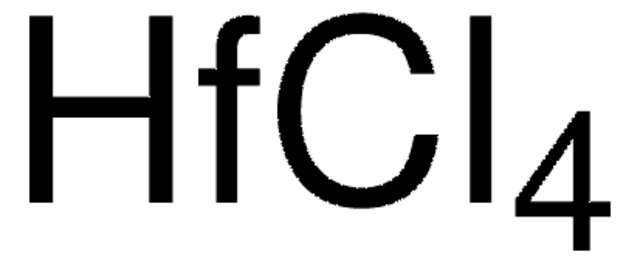
Hafnium(IV) chloride
98%
Synonym(s):
Hafnium tetrachloride, Tetrachlorohafnium
About This Item
Recommended Products
vapor pressure
1 mmHg ( 190 °C)
Quality Level
Assay
98%
form
powder
mp
432 °C (lit.)
solubility
H2O: decomposes(lit.)
SMILES string
Cl[Hf](Cl)(Cl)Cl
InChI
1S/4ClH.Hf/h4*1H;/q;;;;+4/p-4
InChI key
PDPJQWYGJJBYLF-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A precursor in the synthesis of lithium hafnium phosphate, which is a solid electrolyte material used in lithium batteries due to its high ionic conductivity and chemical stability.
- A catalyst in the acetalization process of various carbonyl compounds, including aldehydes and ketones.
- A catalyst in the direct ester condensation of carboxylic acids with alcohols.
- A high-capacity cathode material for lithium and sodium-ion batteries.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1
Supplementary Hazards
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In the last two decades, a new method termed solid-state metathesis (SSM) has been developed to synthesize compounds that are often difficult to produce conventionally.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service