All Photos(1)
About This Item
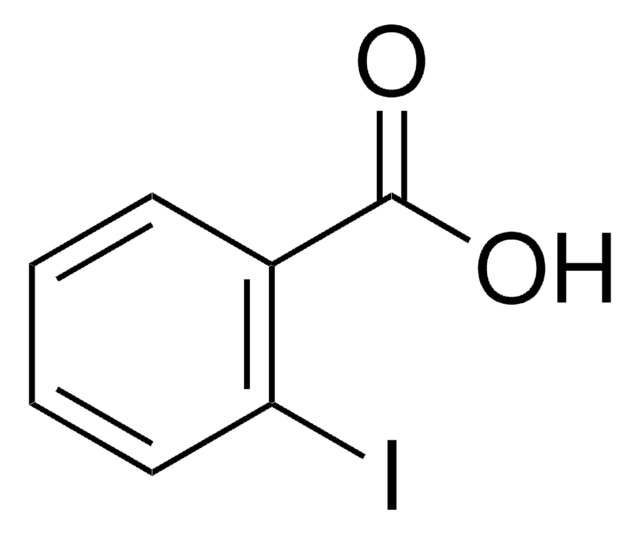
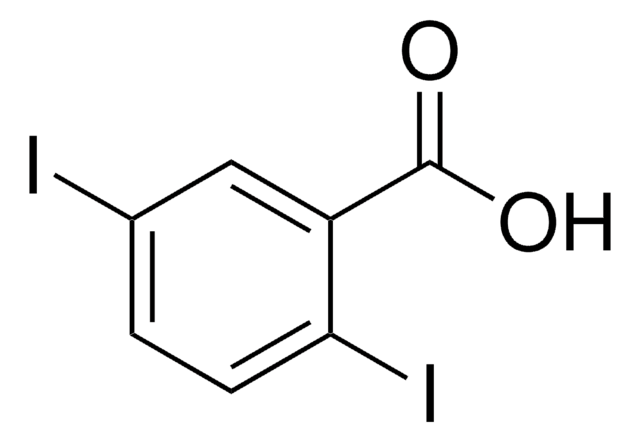
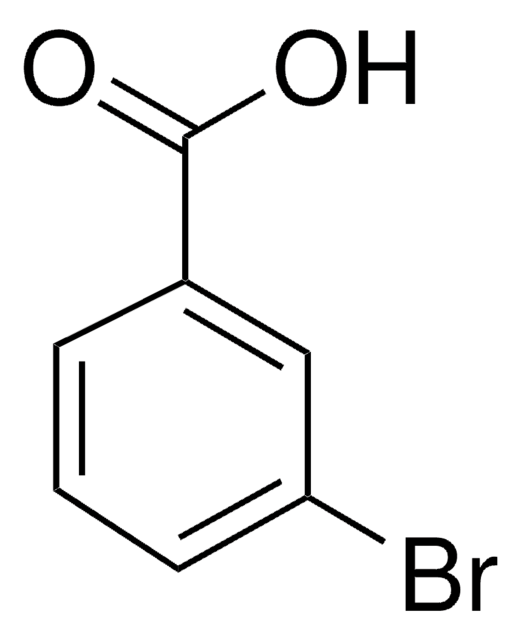
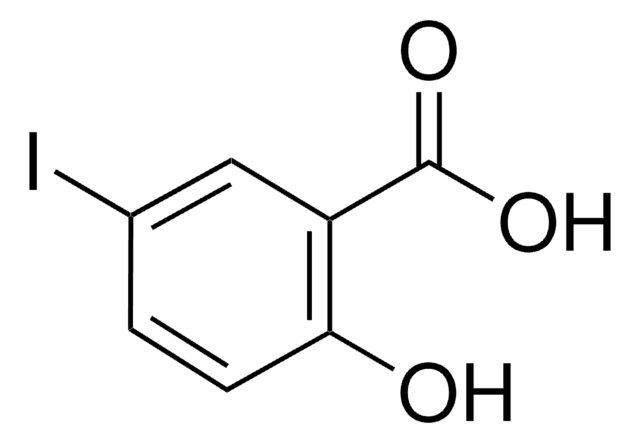
Linear Formula:
IC6H4CO2H
CAS Number:
Molecular Weight:
248.02
Beilstein:
971088
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
185-187 °C (lit.)
SMILES string
OC(=O)c1cccc(I)c1
InChI
1S/C7H5IO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,(H,9,10)
InChI key
KVBWBCRPWVKFQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Iodobenzoic acid is added as UV absorbing background electrolyte in separation of uncharged cyclodextrins and their derivatives by capillary electrophoresis.
Application
3-Iodobenzoic acid was used in solid phase synthesis of γ-turn mimetic library.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A γ-Turn Mimetic Library: Development and Production.
Kocis P, et al.
High-Throughput Synthesis: Principles and Practices, 65-65 (2010)
M Pumera et al.
Fresenius' journal of analytical chemistry, 369(7-8), 666-669 (2001-05-24)
A fast and simple capillary electrophoretic method suitable for the determination of native alpha-, beta-, gamma-cyclodextrins, their randomly substituted tert-butyl derivatives (average degree of substitution 3.8-4.4), heptakis (2,6-di-O-methyl)- and heptakis (2,3,6-tri-O-methyl)-beta-cyclodextrin was developed. Naphthyl-2-sulfonic acid (2-NSA), 3-iodobenzoic acid (3-IBA) and
Negative inotropic effects of Na-salicylate and three congeners on the guinea-pig Langendorff heart.
H Brasch
Archives internationales de pharmacodynamie et de therapie, 262(2), 242-249 (1983-04-01)
In guinea-pig Langendorff hearts, Na-salicylate (1.9, 3.8 and 7.6 mmol/l) concentration-dependently reduced the contractile force (--9.1, --51.0 and --75.1%, respectively) and the coronary resistance. The influence of the uncoupling agent 2.4-dinitrophenol (0.02 mmol/l) was comparable to that of the largest
Pablo Wessig et al.
Molecules (Basel, Switzerland), 18(1), 1314-1324 (2013-01-23)
Various 1,6- and 1,8-naphthalenophanes were synthesized by using the Photo-Dehydro-Diels-Alder (PDDA) reaction of bis-ynones. These compounds are easily accessible from ω-(3-iodophenyl)carboxylic acids in three steps. The obtained naphthalenophanes are axially chiral and the activation barrier for the atropisomerization could be
G Vaidyanathan et al.
Bioconjugate chemistry, 1(6), 387-393 (1990-11-01)
We have previously shown that use of N-succinimidyl 3-iodobenzoate (SIB) for radioiodination of monoclonal antibodies (MAbs) decreases the loss of radioiodine in vivo compared to MAbs labeled by using conventional methods. Herein, the synthesis of N-succinimidyl 2,4-dimethoxy-3-(trialkylstannyl)benzoates (alkyl = Me
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service