12635
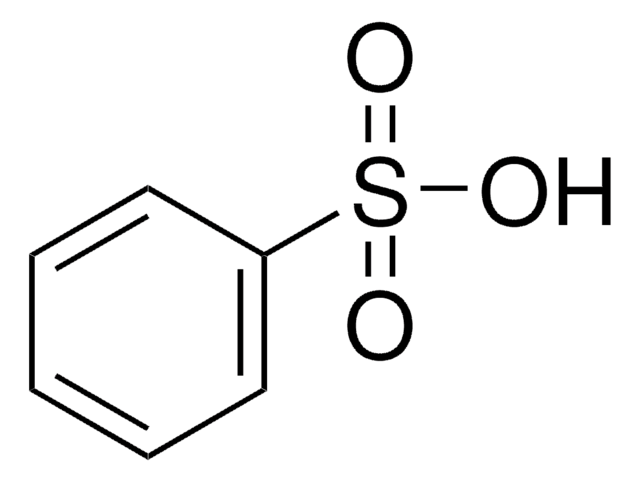
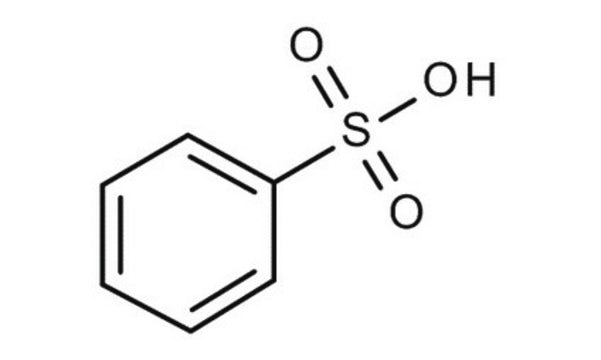
Benzenesulfonic acid
98.0% (T)
Synonym(s):
Phenylsulfonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SO3H
CAS Number:
Molecular Weight:
158.18
Beilstein:
742513
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98.0% (T)
form
solid
impurities
≤1.0% water
SMILES string
OS(=O)(=O)c1ccccc1
InChI
1S/C6H6O3S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H,(H,7,8,9)
InChI key
SRSXLGNVWSONIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzenesulfonic acid (BSA), also known as phenylsulfonic acid, is the simplest aromatic sulfonic acid. It is also used as a catalyst for Michael′s reactions and in the oxidative alkenylation process.
Application
Benzenesulfonic acid is an aryl sulfonic acid that can be used:
- As an oxidizing agent in the synthesis of Fe(III) benzenesulfonate.
- As a catalyst during the vapor-phase nitration of benzene.
- As a reagent in the synthesis of organic salts.
- To form an organic salt with 5,7-dimethyl-1,8-naphthyridine-2-amine, that can be a synthon for developing supramolecular structures.
- As a dopant for the polymerization of pyrrole to form poly(pyrrole), a conducting polymer useful in the development of flexible capacitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Supramolecular salts of 5, 7-dimethyl-1, 8-naphthyridine-2-amine and acids through classical H-Bonds and other intermolecular interactions.
Dong L
Journal of Molecular Structure, 1153, 311-323 (2018)
Durable Flexible Supercapacitors Utilizing the Multifunctional Role of Ionic Liquids.
Lorenzo M
Energy Technology, 6(1), 196-204 (2018)
David Scholz et al.
ChemSusChem, 11(13), 2189-2201 (2018-05-08)
The deactivation pathways of sulfonated carbon catalysts prepared from different carbons were studied during the aqueous-phase hydrolysis of cellobiose under continuous-flow conditions. The sulfonation of carbon materials with a low degree of graphitization introduced sulfonic acid groups that are partially
Bo Ram Lee et al.
Journal of colloid and interface science, 331(1), 55-59 (2008-12-09)
Sulfur hexafluoride (SF(6)) has been widely used in a variety of industrial processes, but it is one of the most potent greenhouse gases. For this reason, it is necessary to separate or collect it from waste gas streams. One separation
Yanlong Gu et al.
Organic letters, 9(2), 175-178 (2007-01-16)
A neutral catalytic system for Michael reactions of indoles has been developed by combining silica-supported benzenesulfonic acid sodium salt with hydrophobic ionic liquid in water. An efficient hydrophobic environment could be created on the surface of the silica-sodium material under
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service