All Photos(2)
About This Item
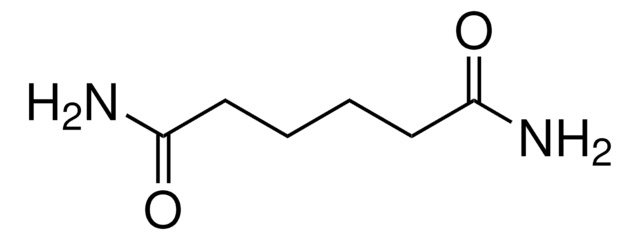
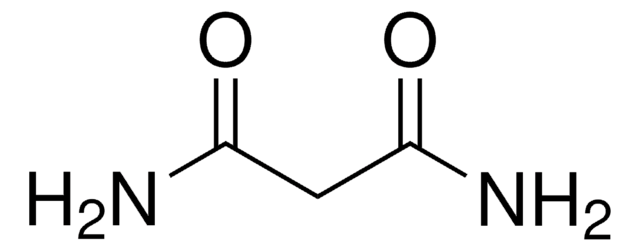
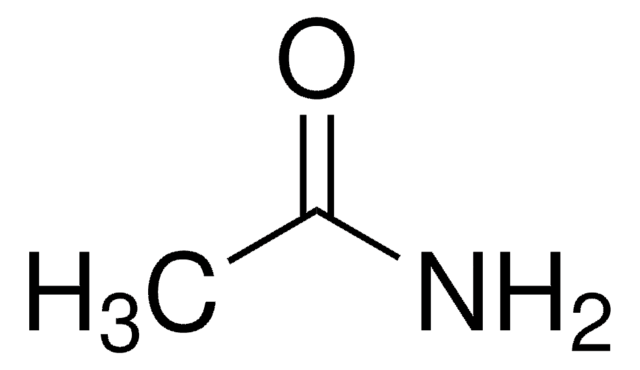
Linear Formula:
NH2COCH2CH2CONH2
CAS Number:
Molecular Weight:
116.12
Beilstein:
1753983
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
260-265 °C (dec.) (lit.)
solubility
cold water: soluble 220 part
boiling water: soluble 9 part
alcohol: insoluble
diethyl ether: insoluble
SMILES string
NC(=O)CCC(N)=O
InChI
1S/C4H8N2O2/c5-3(7)1-2-4(6)8/h1-2H2,(H2,5,7)(H2,6,8)
InChI key
SNCZNSNPXMPCGN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Succinamide has been used in the characterization of a novel biuret hydrolase from the cysteine hydrolase superfamily. It was used as nitrogen supplement in the culture medium of Scenedesmus obliquus and green algae.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Birgit T Priest et al.
Biochemistry, 43(30), 9866-9876 (2004-07-28)
Sodium channel blockers are used clinically to treat a number of neuropathic pain conditions, but more potent and selective agents should improve on the therapeutic index of currently used drugs. In a high-throughput functional assay, a novel sodium channel (Na(V))
Petras P Dzeja et al.
American journal of physiology. Heart and circulatory physiology, 284(4), H1048-H1056 (2003-04-02)
Modulation of mitochondrial respiratory chain, dehydrogenase, and nucleotide-metabolizing enzyme activities is fundamental to cellular protection. Here, we demonstrate that the potassium channel opener diazoxide, within its cardioprotective concentration range, modulated the activity of flavin adenine dinucleotide-dependent succinate dehydrogenase with an
T Watanabe et al.
Chemical & pharmaceutical bulletin, 45(6), 996-1007 (1997-06-01)
A series of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one derivatives containing the succinamide skeleton has been synthesized and evaluated for M1, M2 and M3 muscarinic receptor binding affinities (in vitro) and M2 and M3 muscarinic receptor antagonistic activities (in vivo). Some of them showed higher
Xiaobin Zhang et al.
Drug delivery, 11(5), 301-309 (2005-03-04)
To investigate the effect of RMP-7 and its derivative on drug transport across blood brain barrier (BBB), RMP-7 and DSPE-PEG-NHS [1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-n-[poly(ethyleneglycol)]-hydroxy succinamide, PEG M 3400] were conjugated under mild conditions and the reaction ratio was determined using MALDI-TOF-MS (matrix-assisted laser
Olaf F A Larsen et al.
Proceedings of the National Academy of Sciences of the United States of America, 102(38), 13378-13382 (2005-09-10)
Femtosecond 2D-IR spectroscopy has been used to study the structure of a [2]rotaxane composed of a benzylic amide macrocycle that is mechanically interlocked onto a succinamide-based thread. Both the macrocycle and the thread contain carbonyl groups, and by determining the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service