1271700
USP
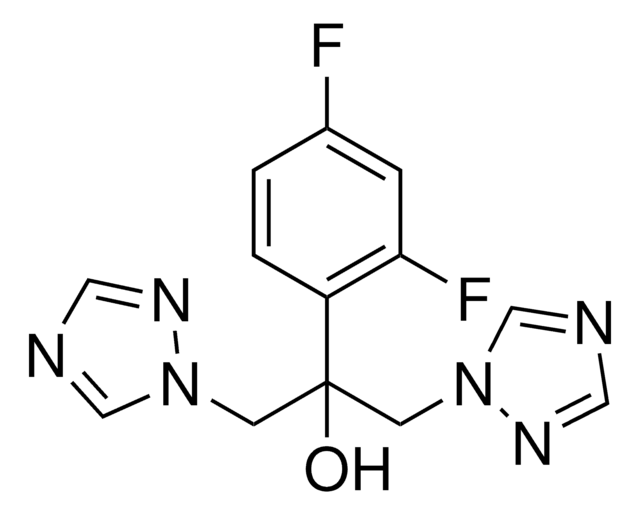
Fluconazol
United States Pharmacopeia (USP) Reference Standard
Synonym(e):
Fluconazol, 2-(2,4-Difluorphenyl)-1,3-bis-(1H-1,2,4-triazol-1-yl)-propan-2-ol
About This Item
Empfohlene Produkte
Qualität
pharmaceutical primary standard
API-Familie
fluconazole
Hersteller/Markenname
USP
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
SMILES String
FC1=CC(F)=C(C(CN2N=CN=C2)(O)CN3N=CN=C3)C=C1
InChI
1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2
InChIKey
RFHAOTPXVQNOHP-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Enhanced antifungal activity of fluconazole: A study optimized fluconazole-embedded transfersomal gel, demonstrating improved antifungal activity and compatibility. This research is crucial for enhancing fluconazole′s efficacy against resistant fungal strains, making it a pivotal tool in the fight against fungal infections (Cheng et al., 2024).
- Antifungal mechanisms against drug-resistant strains: Research on the antifungal activity of a trypsin inhibitor from chia seeds against fluconazole-resistant Candida species assessed its potential as a novel therapeutic approach. This study contributes valuable insights into natural compounds enhancing fluconazole′s effectiveness, crucial for developing alternative antifungal therapies (Nogueira et al., 2024).
- Advancements in fungal pathogenesis: The isolation and identification of Wickerhamiella tropicalis from blood culture using MALDI-MS highlight innovative diagnostic techniques that enhance the understanding of fungal pathogenesis. This research is essential for advancing microbial diagnostics and tailoring treatments to combat invasive fungal infections effectively (Takei et al., 2024).
Biochem./physiol. Wirkung
Hinweis zur Analyse
Sonstige Hinweise
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Chronic 3 - Lact. - Repr. 1B
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.