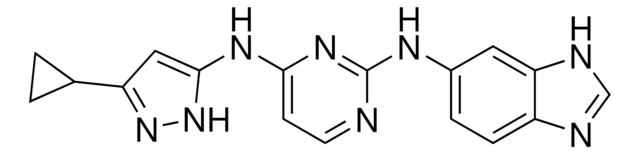
SML0409
STF-083010
≥98% (HPLC)
Synonym(e):
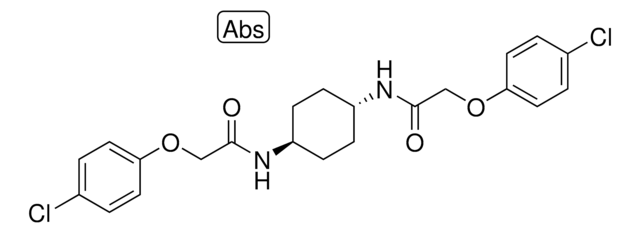
N-[(2-Hydroxy-1-naphthalenyl)methylene]-2-thiophenesulfonamide
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
light yellow to yellow-green
Löslichkeit
DMSO: 5 mg/mL (clear solution)
Lagertemp.
−20°C
SMILES String
OC1=C(/C=N/S(C2=CC=CS2)(=O)=O)C3=C(C=CC=C3)C=C1
InChI
1S/C15H11NO3S2/c17-14-8-7-11-4-1-2-5-12(11)13(14)10-16-21(18,19)15-6-3-9-20-15/h1-10,17H/b16-10+
InChIKey
TVIVJHZHPKNDAQ-MHWRWJLKSA-N
Anwendung
- in a study to investigate the potential anti-lipotoxic effect of nicotinamide and to elucidate underlying mechanism(s)
- as IRE1a inhibitor to study its effect on NOS 2 expression and investigate the underlying mechanisms in proinflammatory gene expression in astrocytes
- to block endogenous XBP1 cleavage for one hour prior to palmitate exposure in order to examine whether inositol?requiring enzyme 1α (IRE1α ) activation is implicated in palmitate cytotoxicity
Biochem./physiol. Wirkung
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![PERK-Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)