O4636
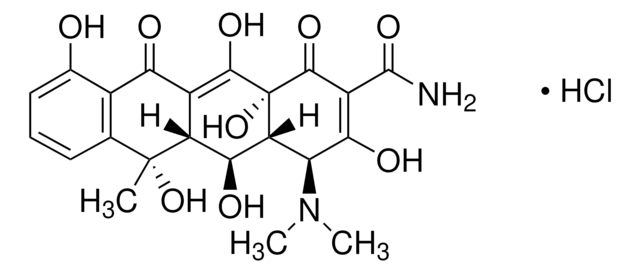
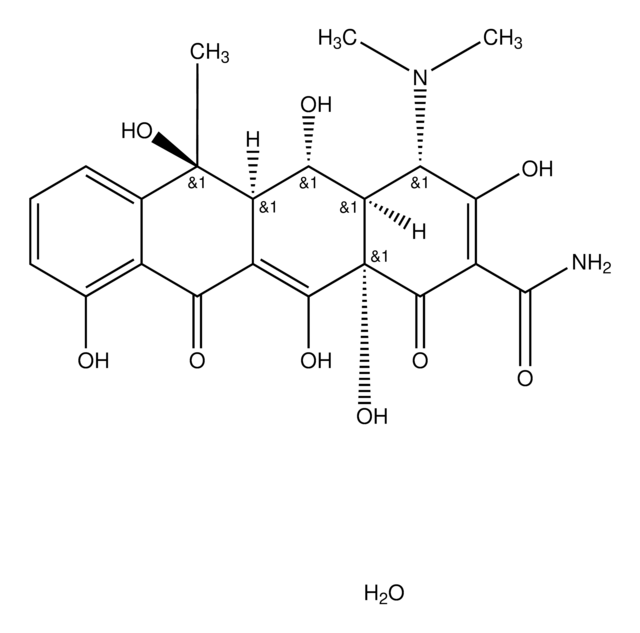
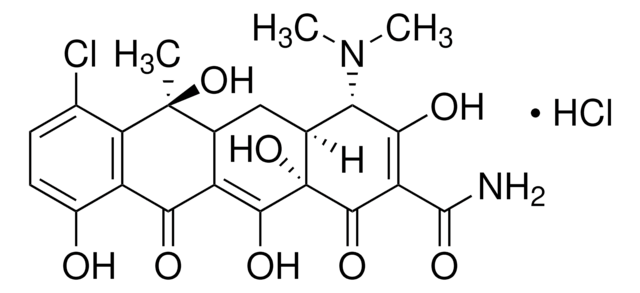
Oxytetracyclin Dihydrat
Assay (anhydrous basis) 95.0%-102.0%
Synonym(e):
5-Hydroxytetracycline dihydrate, Oxytetracycline, Oxytetracycline Anhydrous
About This Item
Empfohlene Produkte
Qualitätsniveau
Wirkungsspektrum von Antibiotika
Gram-negative bacteria
Gram-positive bacteria
Wirkungsweise
protein synthesis | interferes
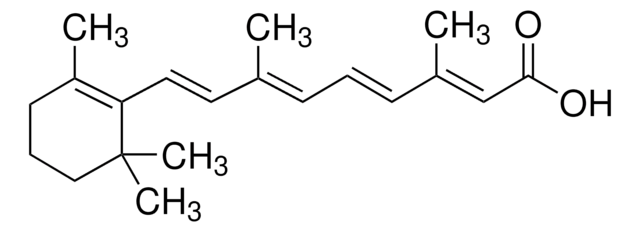
SMILES String
O.[H][C@@]12[C@@H](O)[C@@]3([H])C(C(=O)c4c(O)cccc4[C@@]3(C)O)=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C
InChI
1S/C22H24N2O9.H2O/c1-21(32)7-5-4-6-8(25)9(7)15(26)10-12(21)17(28)13-14(24(2)3)16(27)11(20(23)31)19(30)22(13,33)18(10)29;/h4-6,12-14,17,25,27-29,32-33H,1-3H3,(H2,23,31);1H2/t12-,13-,14+,17+,21-,22+;/m1./s1
InChIKey
IBZHEBHGZFICKS-IFLJXUKPSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Mode of Action: Inhibits protein synthesis (elongation) by preventing binding of aminoacyl-tRNA to the 30S subunit.
Antimicrobial spectrum: Gram-negative and Gram-positive bacteria.
Mode of Resistance: Active efflux, ribosome protection, tetracycline inactivation.
Hinweis zur Analyse
Sonstige Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
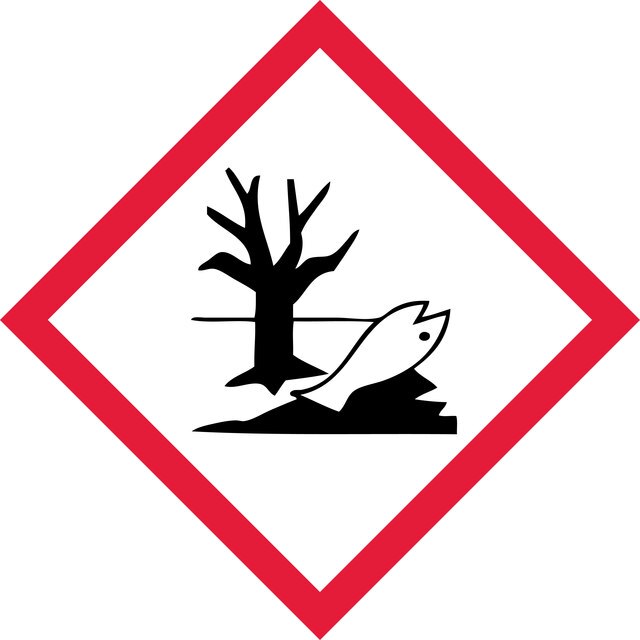
Aquatic Chronic 2 - Repr. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.