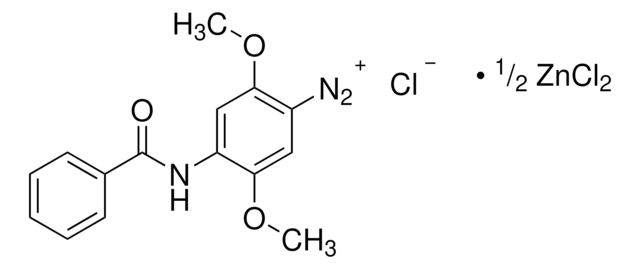
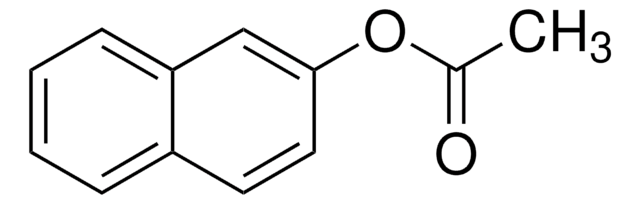
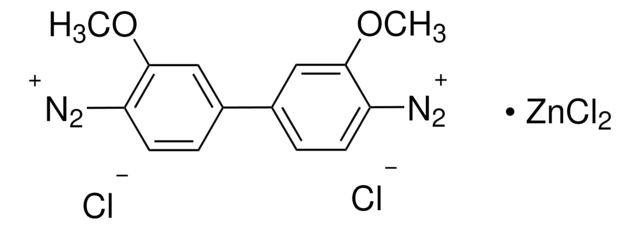
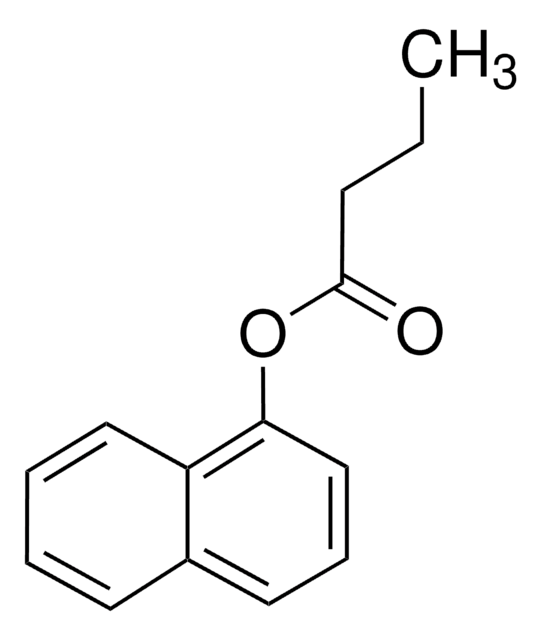
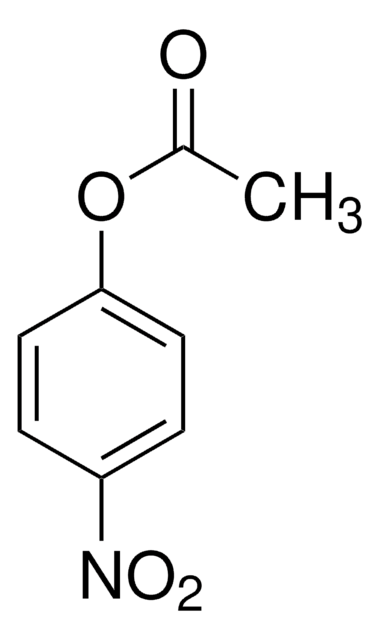
N8505
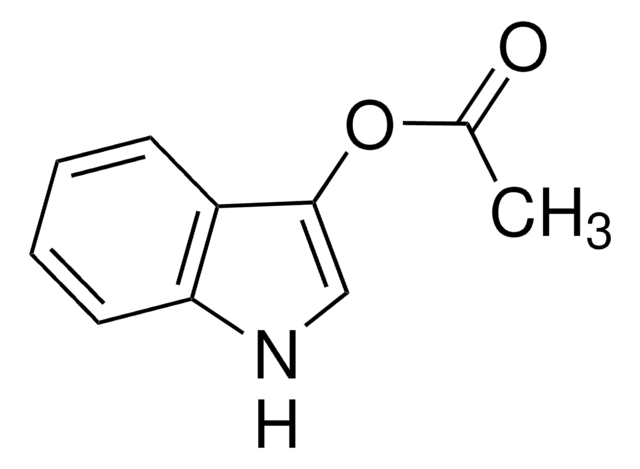
1-Naphthylacetat
≥98% (C)
Synonym(e):
α-Naphthyl-acetat
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (C)
Form
crystals
mp (Schmelzpunkt)
43-46 °C (lit.)
Lagertemp.
−20°C
SMILES String
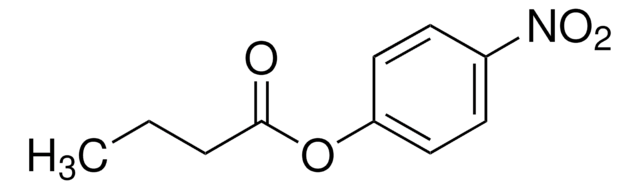
CC(=O)Oc1cccc2ccccc12
InChI
1S/C12H10O2/c1-9(13)14-12-8-4-6-10-5-2-3-7-11(10)12/h2-8H,1H3
InChIKey
VGKONPUVOVVNSU-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
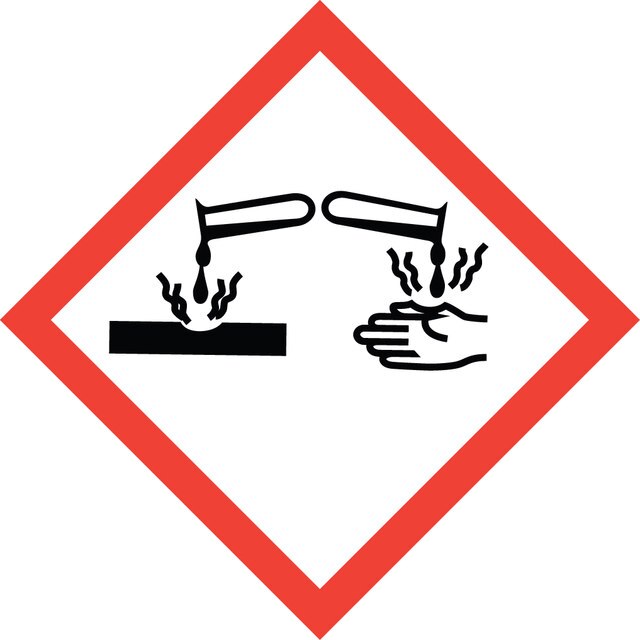
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Dam. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F
Flammpunkt (°C)
113 °C
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.