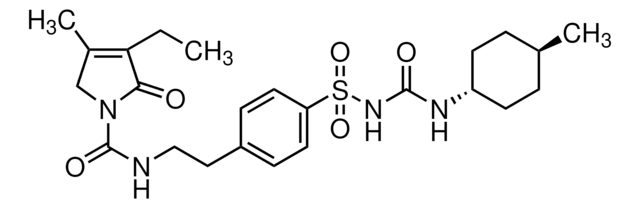
G117
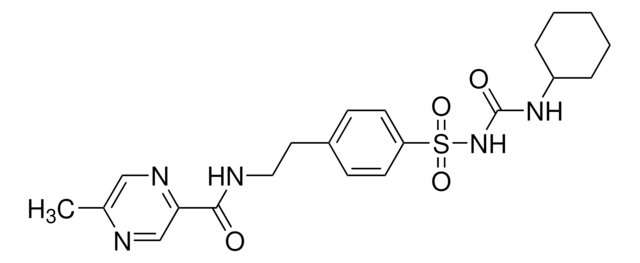
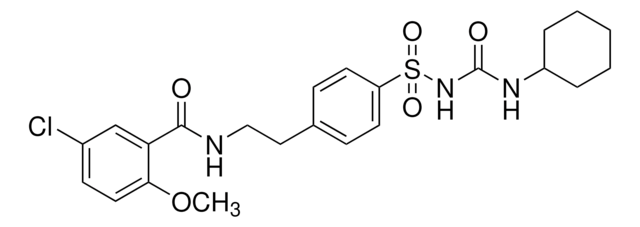
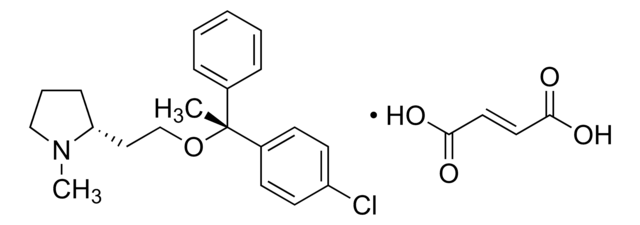
Glipizid
solid
Synonym(e):
1-Cyclohexyl-3-{4-[2-(5-methylpyrazin-2-carboxamido)-ethyl]-phenylsulfonyl}-harnstoff
About This Item
Empfohlene Produkte
Form
solid
Qualitätsniveau
Farbe
white
Löslichkeit
methanol: 1.9 mg/mL
DMSO: 48 mg/mL
SMILES String
Cc1cnc(cn1)C(=O)NCCc2ccc(cc2)S(=O)(=O)NC(=O)NC3CCCCC3
InChI
1S/C21H27N5O4S/c1-15-13-24-19(14-23-15)20(27)22-12-11-16-7-9-18(10-8-16)31(29,30)26-21(28)25-17-5-3-2-4-6-17/h7-10,13-14,17H,2-6,11-12H2,1H3,(H,22,27)(H2,25,26,28)
InChIKey
ZJJXGWJIGJFDTL-UHFFFAOYSA-N
Angaben zum Gen
human ... ABCC8(6833) , KCNJ1(3758) , KCNJ11(3767)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- chick embryo yolk sac membrane (YSM) and chorioallantoic membrane (CAM) assay
- MCF-7 breast cancer assay on chorioallantoic membrane (CAM)
- metastasis assays
- rat aortic ring assay
- in vivo matrigel plug assay
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
- flow cytometry
- western blotting
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Glucose metabolism is regulated by the opposing actions of insulin and glucagon. Insulin is released from pancreatic ß cells in response to high blood glucose levels and regulates glucose metabolism through its actions on muscle, liver, and adipose tissue.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.