B6938
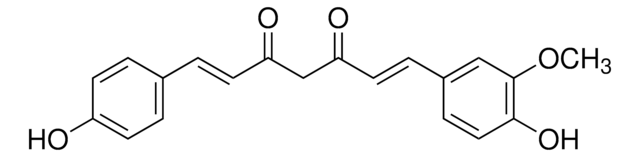
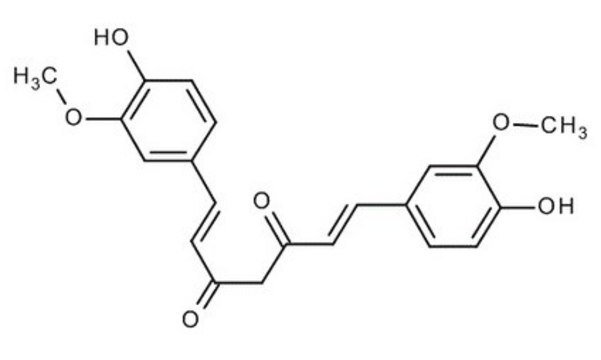
Bisdemethoxycurcumin
≥98% (HPLC), solid
Synonym(e):
(1E,6E)-1,7-bis(4-hydroxyphenyl)hepta-1,6-diene-3,5-dione
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
solid
Löslichkeit
DMSO: ≥20 mg/mL
Lagertemp.
2-8°C
SMILES String
Oc1ccc(cc1)\C=C\C(=O)CC(=O)\C=C\c2ccc(O)cc2
InChI
1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2/b11-5+,12-6+
InChIKey
PREBVFJICNPEKM-YDWXAUTNSA-N
Anwendung
- to test its inhibitory effect on cell cycle and mitochondrial function in gastric adenocarcinoma cells
- to test it neuroprotective role against lead (Pb) induced toxicity in dopaminergic and noradrenergic systems of Meriones shawi
- as a standard for calibration curve generation to quantify plasma curcuminoids using high-performance liquid chromatography-diode array detection (HPLC-DAD) and ultraviolet (UV)
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.