A9891
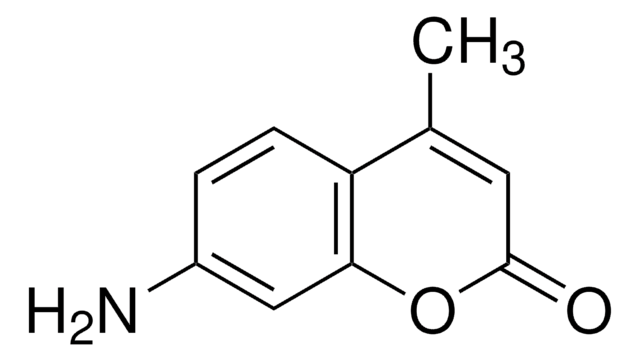
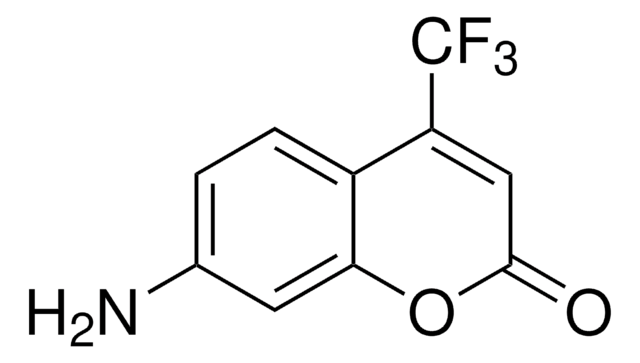
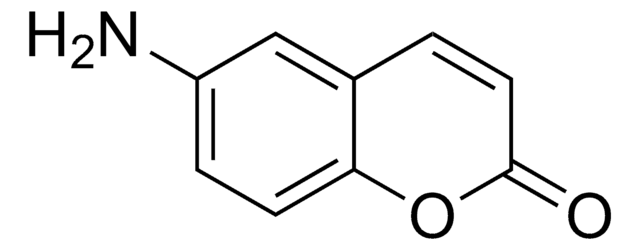
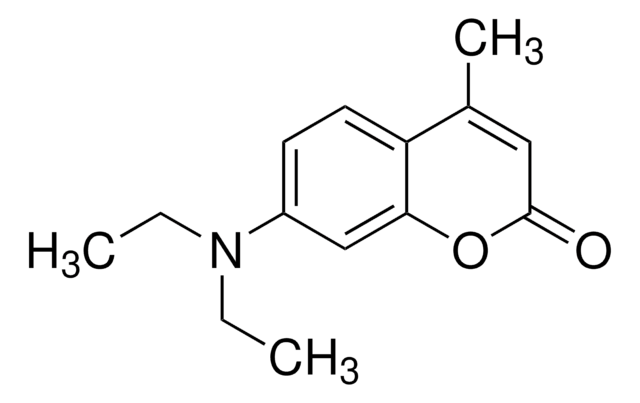
7-Amino-4-methylcumarin
Chromophore for substrates
Synonym(e):
Coumarin 120
About This Item
Empfohlene Produkte
Qualitätsniveau
Beschreibung
chromophore for enzyme substrates
Assay
≥98% (HPLC)
Form
powder
mp (Schmelzpunkt)
223-226 °C (lit.)
Löslichkeit
acetone: 10 mg/mL, clear, colorless to yellow
Fluoreszenz
λex 365 nm; λem 440 nm in ethanol(lit.)
Lagertemp.
2-8°C
SMILES String
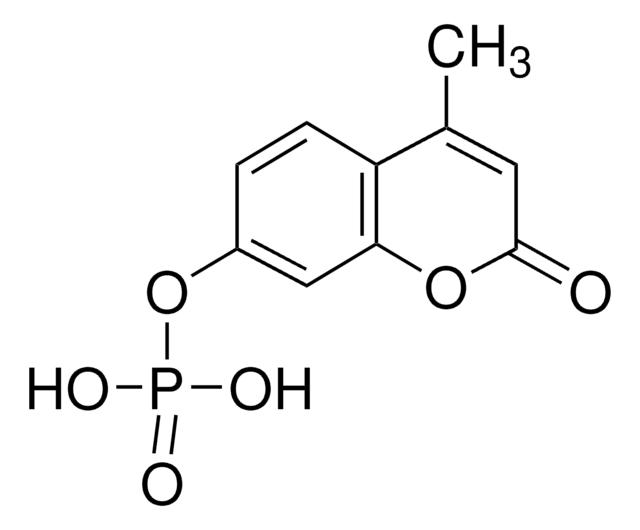
CC1=CC(=O)Oc2cc(N)ccc12
InChI
1S/C10H9NO2/c1-6-4-10(12)13-9-5-7(11)2-3-8(6)9/h2-5H,11H2,1H3
InChIKey
GLNDAGDHSLMOKX-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- to determine cathepsin B like- activity
- as a substrate for leucine aminopeptidase (LAP)
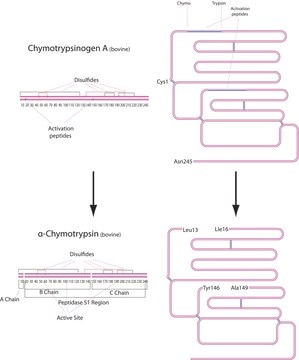
- in chymotryptic assay
- as a standard to detect the activation of caspase-3 during
- sanguinarine-induced damage
- protection by human DEAD-box DDX3
Biochem./physiol. Wirkung
Angaben zur Herstellung
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
This procedure applies to all products that have a specification for Cathepsin B activity determined by the liberation of 7-amino-4-methylcoumarin from Z-Arg-Arg 7-amido-4-methylcoumarin.
Mass Spectrometry of Glycans, method comparison and products
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.