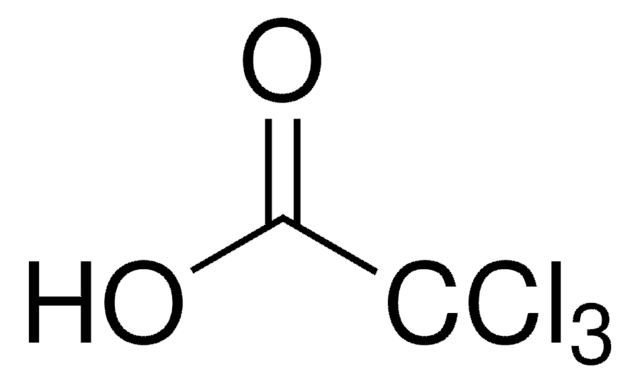
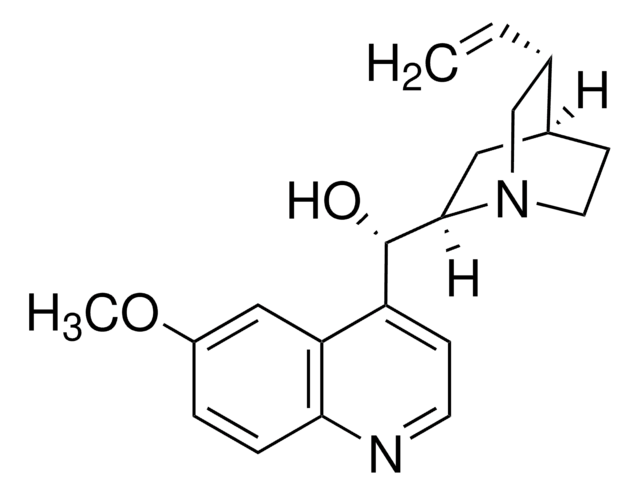
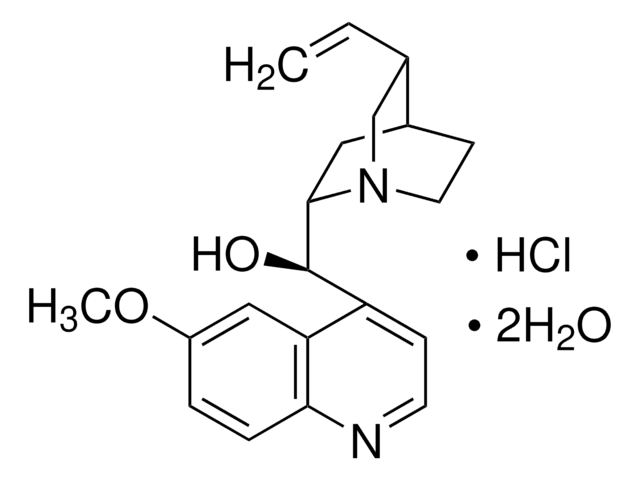
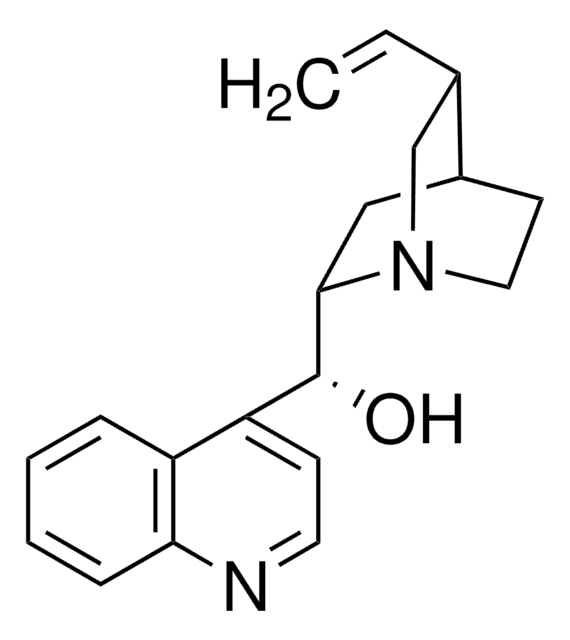
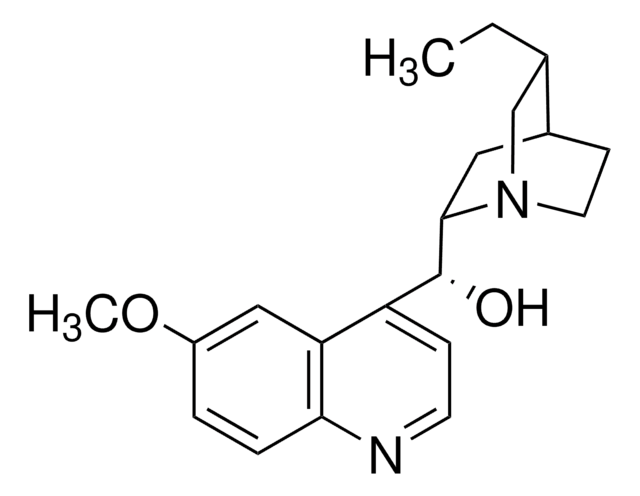
22620
Chinin
suitable for fluorescence, anhydrous, ≥98.0% (dried material, NT)
Synonym(e):
6′-Methoxycinchonidin
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98.0% (dried material, NT)
Form
powder
Optische Aktivität
[α]20/D −126±5°, c = 1% in chloroform
Verunreinigungen
≤5% dihydroquinine (HPLC)
Verlust
≤1% loss on drying, 110 °C
mp (Schmelzpunkt)
173-175 °C (lit.)
Löslichkeit
H2O: soluble
Fluoreszenz
λex 347 nm; λem 448 nm in 0.5 M sulfuric acid
Eignung
suitable for fluorescence
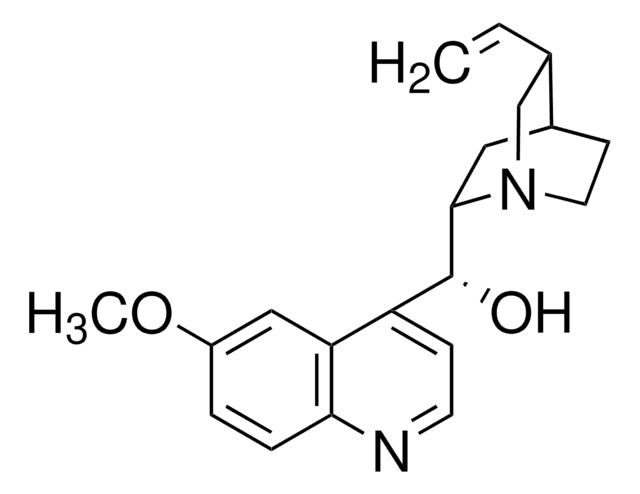
SMILES String
COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1
InChIKey
LOUPRKONTZGTKE-WZBLMQSHSA-N
Angaben zum Gen
human ... ABCB1(5243) , CYP2C9(1559) , CYP2D6(1565)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- To study its in vitro antimalarial activity in combination with omeprazole.
- To analyze its effect on viscosity and friction of saliva.
- As a test agent to study its impact on the accumulation of the fluorescent P-glycoprotein (Pgp) substrates in P-glycoprotein overexpressing breast cancer cells.
- To study its influence on the pyramidal cell intrinsic properties, extracellular potassium transients, and epileptiform activity in vitro.
- As a reference compound to identify alkaloids by phytochemical screening of Deianira erubescens, Strychnos pseudoquina and Remijia ferruginea plants.
Biochem./physiol. Wirkung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.