PHR1978
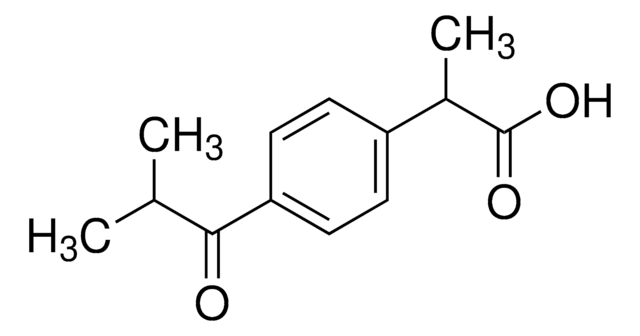
(2RS)-2-(4-Isobutyrylphenyl)propionsäure
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
αα-Methyl-4-(2-methyl-1-oxopropyl)benzolessigsäure, (2RS)-2-[4-(2-Methylpropanoyl)phenyl]propansäure, 1-Oxoibuprofen
About This Item
Empfohlene Produkte
Qualität
certified reference material
pharmaceutical secondary standard
Qualitätsniveau
Agentur
traceable to USP 1335596
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
pkg of 50 mg
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-30°C
SMILES String
OC(C(C)C1=CC=C(C(C(C)C)=O)C=C1)=O
InChI
1S/C13H16O3/c1-8(2)12(14)11-6-4-10(5-7-11)9(3)13(15)16/h4-9H,1-3H3,(H,15,16)
InChIKey
RCINGEKPKJQCMO-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Prediction of Potential Toxicity and Side Effect Protein Targets: Research exploring the use of Ibuprofen Related Compound J as part of a ligand-protein inverse docking approach aimed at predicting potential toxicity and side effect protein targets. This innovative method enhances drug safety profiles by identifying off-target interactions early in the drug development process, serving as a critical tool for pharmaceutical research and ensuring the development of safer therapeutic agents (Chen and Ung, 2001).
Hinweis zur Analyse
Sonstige Hinweise
Fußnote
Empfohlene Produkte
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.