N9890
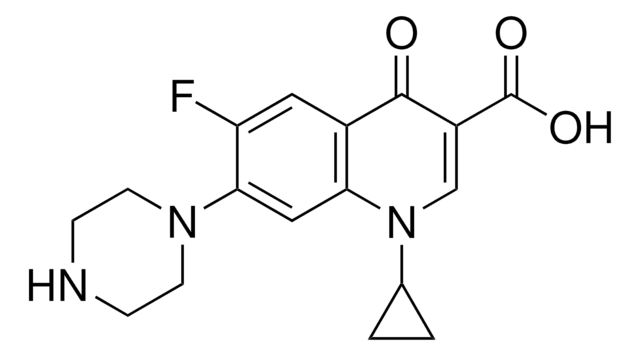
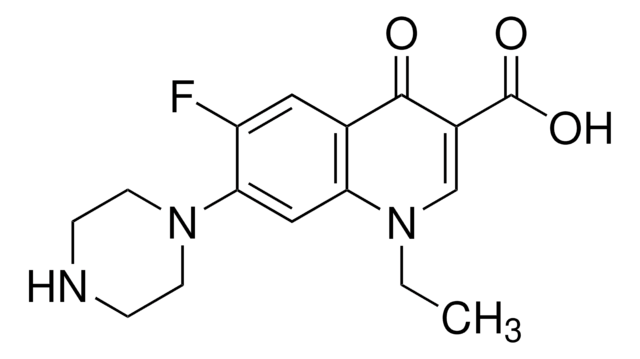
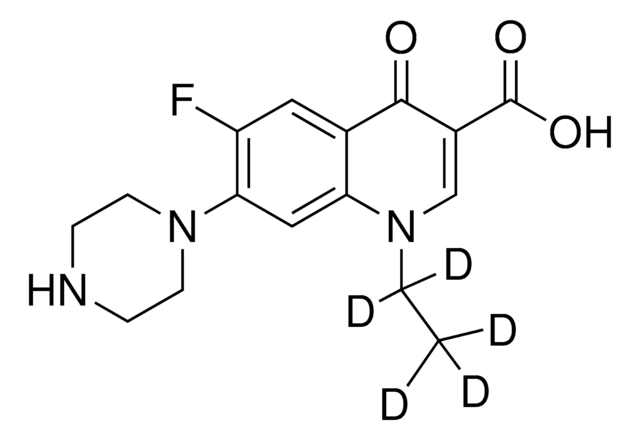
Norfloxacin
analytical standard, ≥98% (TLC)
Synonym(e):
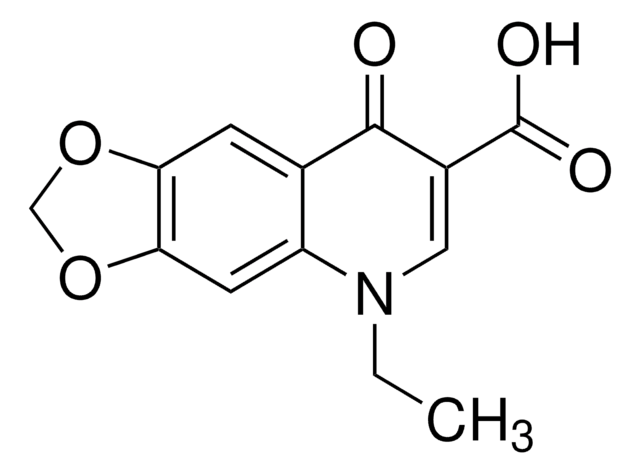
1-Ethyl-6-fluor-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-chinolincarbonsäure, 1-Ethyl-6-fluor-1,4-dihydro-4-oxo-7-piperazino-3-chinolincarbonsäure
About This Item
Empfohlene Produkte
Qualität
analytical standard
Qualitätsniveau
Agentur
EPA 1694
Assay
≥98% (TLC)
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Anwendung(en)
clinical testing
Format
neat
Lagertemp.
2-8°C
SMILES String
CCN1C=C(C(O)=O)C(=O)c2cc(F)c(cc12)N3CCNCC3
InChI
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
InChIKey
OGJPXUAPXNRGGI-UHFFFAOYSA-N
Angaben zum Gen
human ... CYP1A2(1544)
rat ... Gabra1(29705)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Wirkungsweise: hemmt die bakterielle DNA-Replikation
Antimikrobielles Spektrum: Gram-negative Bakterien, weniger effektiv bei Gram-positiven Bakterien
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.