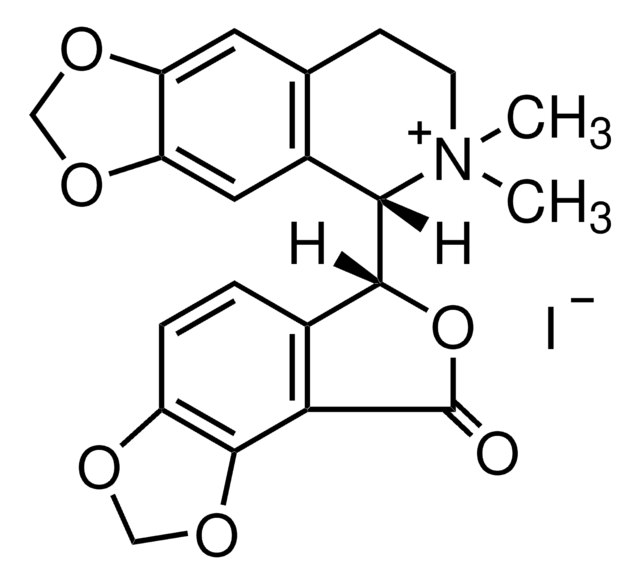
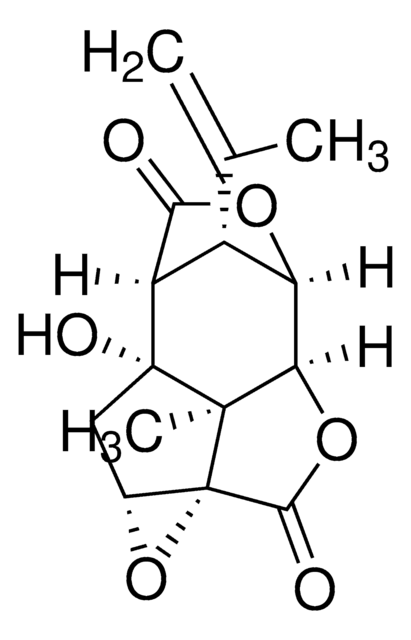
14340
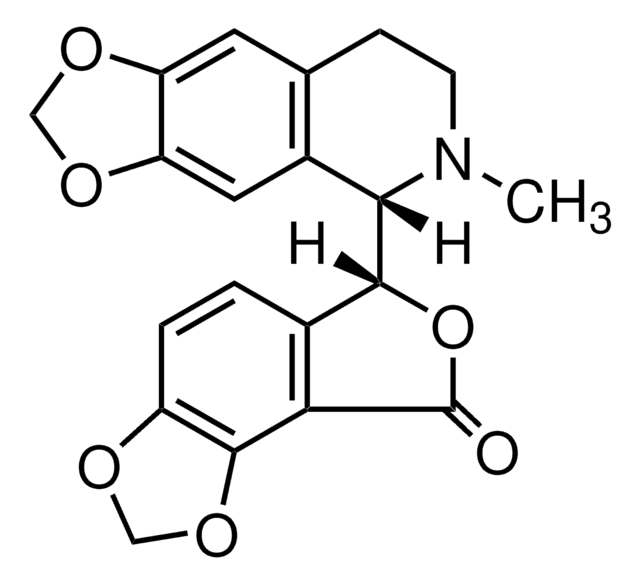
(+)-Bicucullin
≥97.0% (TLC)
Synonym(e):
Bucucullin
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥97.0% (TLC)
Form
powder
Optische Aktivität
[α]20/D +126±6°, c = 1% in chloroform
mp (Schmelzpunkt)
193-197 °C
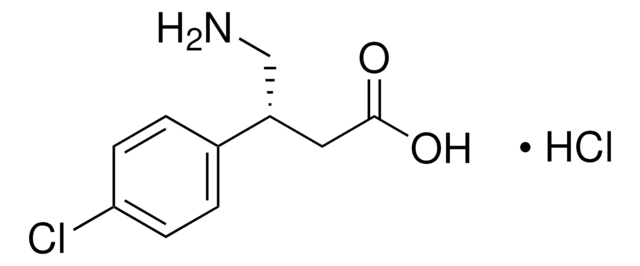
SMILES String
[H][C@]1(OC(=O)c2c3OCOc3ccc12)[C@@]4([H])N(C)CCc5cc6OCOc6cc45
InChI
1S/C20H17NO6/c1-21-5-4-10-6-14-15(25-8-24-14)7-12(10)17(21)18-11-2-3-13-19(26-9-23-13)16(11)20(22)27-18/h2-3,6-7,17-18H,4-5,8-9H2,1H3/t17-,18+/m0/s1
InChIKey
IYGYMKDQCDOMRE-ZWKOTPCHSA-N
Angaben zum Gen
rat ... Gabra2(29706)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- as a compound to compare pharmacodynamics and network activity profiles of conolidine/cannabidiol
- to study the effects of chronic caffeine administration on the function of GABAA receptor
- to isolate N-methyl-D-aspartate receptor (NMDAR)-specific evoked and miniature excitatory postsynaptic currents (eEPSCs and mEPSCs) in neurons of rats
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Yeast is one of the most important microorganisms known and utilised by mankind. Ancient Middle Eastern civilisations used the organism to bake bread and to produce mead, beer and wine.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.