09658
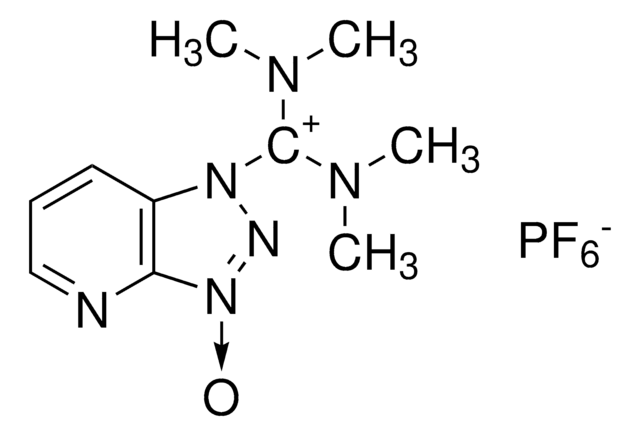
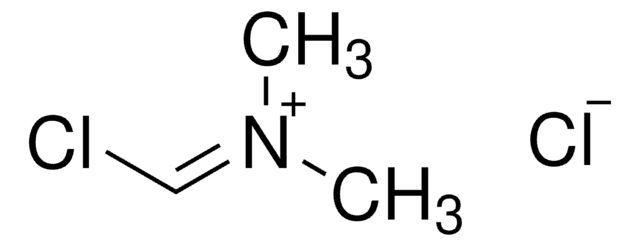
Chlor-N,N,N′,N′-tetramethylformamidinium-hexafluorophosphat
≥98.0% (T)
Synonym(e):
TCFH
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98.0% (T)
Eignung der Reaktion
reaction type: Coupling Reactions
mp (Schmelzpunkt)
99-118 °C
Anwendung(en)
peptide synthesis
Lagertemp.
2-8°C
SMILES String
F[P-](F)(F)(F)(F)F.CN(C)\C(Cl)=[N+](\C)C
InChI
1S/C5H12ClN2.F6P/c1-7(2)5(6)8(3)4;1-7(2,3,4,5)6/h1-4H3;/q+1;-1
InChIKey
CUKNPSDEURGZCO-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
- Onium salts for use in peptide coupling.
- Benzotriazole based uranium reagent, a safer replacement for coupling reagents.
It can also be used as a reagent for the synthesis of:
- Cancer cell cytotoxins.
- Bioconjugation reagents.
Sonstige Hinweise
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.