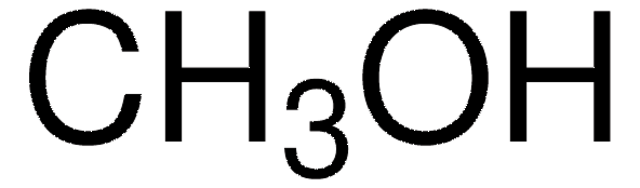02482
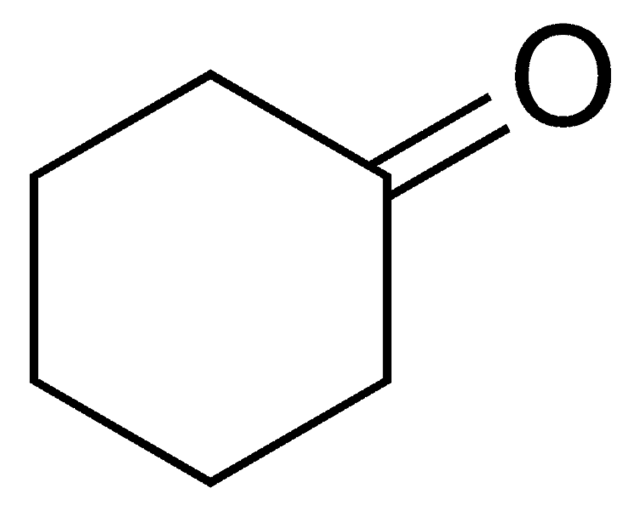
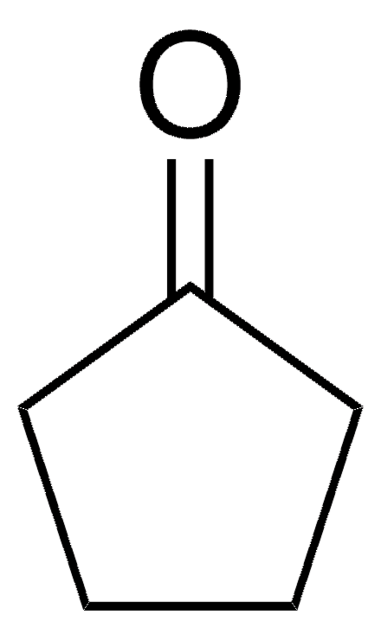
Cyclohexanon
analytical standard
About This Item
Empfohlene Produkte
Qualität
analytical standard
Qualitätsniveau
Dampfdichte
3.4 (vs air)
Dampfdruck
3.4 mmHg ( 20 °C)
Assay
≥99.9% (GC)
Selbstzündungstemp.
788 °F
Haltbarkeit
limited shelf life, expiry date on the label
Expl.-Gr.
1.1 %, 100 °F
9.4 %
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Brechungsindex
n20/D 1.450 (lit.)
n20/D 1.451
bp
155 °C (lit.)
mp (Schmelzpunkt)
−47 °C (lit.)
Dichte
0.947 g/mL at 25 °C (lit.)
Anwendung(en)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
Format
neat
SMILES String
O=C1CCCCC1
InChI
1S/C6H10O/c7-6-4-2-1-3-5-6/h1-5H2
InChIKey
JHIVVAPYMSGYDF-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- vitamin A
- 2-cyclohexylidene cyclohexanone via Aldol condensation reaction
- 2-cyclohexenyl cyclohexanone via Aldol condensation reaction
- bis-(arylmethylidene)cycloalkanones by cross-aldol condensation with aldehydes
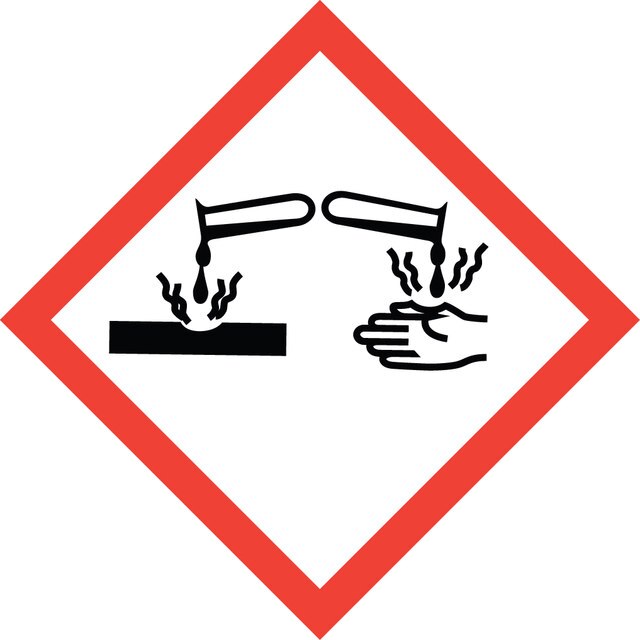
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 1
Flammpunkt (°F)
111.2 °F - closed cup
Flammpunkt (°C)
44 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The aldol condensation reaction is an organic reaction introduced by Charles Wurtz, who first prepared the β-hydroxy aldehyde from acetaldehdye in 1872.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.