00920
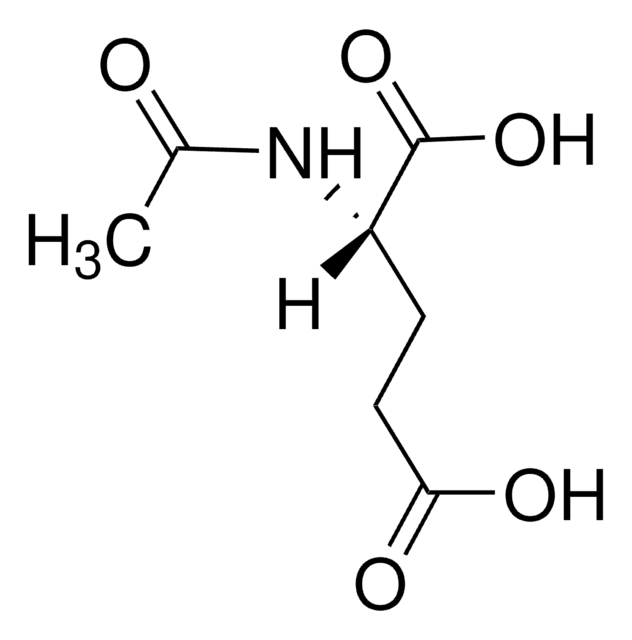
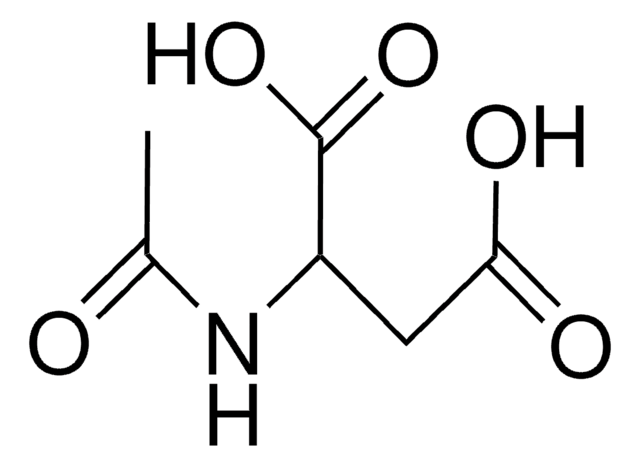
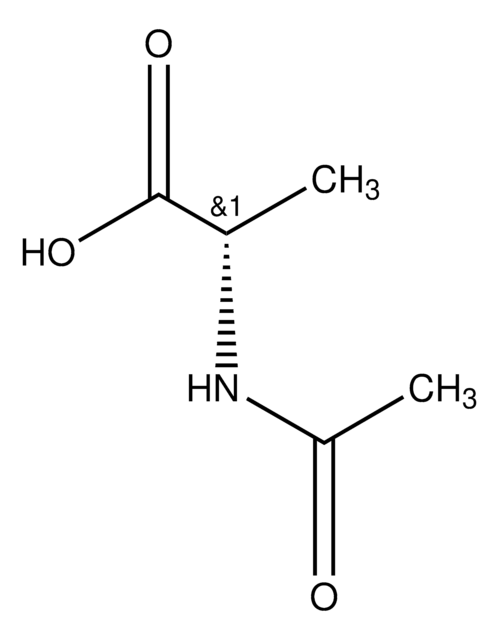
N-Acetyl-L-asparaginsäure
≥99.0% (T)
Synonym(e):
(2S)-2-Acetamidobutanedioic acid, N-Acetyl-S-aspartic acid
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99.0% (T)
Form
powder
Optische Aktivität
[α]20/D +12±1°, c = 2% in 6 M HCl
Eignung der Reaktion
reaction type: solution phase peptide synthesis
Farbe
colorless to white
mp (Schmelzpunkt)
137-140 °C (lit.)
141-146 °C
Anwendung(en)
peptide synthesis
SMILES String
CC(=O)N[C@@H](CC(O)=O)C(O)=O
InChI
1S/C6H9NO5/c1-3(8)7-4(6(11)12)2-5(9)10/h4H,2H2,1H3,(H,7,8)(H,9,10)(H,11,12)/t4-/m0/s1
InChIKey
OTCCIMWXFLJLIA-BYPYZUCNSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Protected homoserine γ-lactones by selective reduction and acid-catalyzed cyclization reaction.
- Racemic amino substituted succinimide derivatives via cyclocondensation reaction.
Sonstige Hinweise
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Inborn errors of metabolism are caused by changes in specific enzymatic reactions and hundreds of different such alterations, which affect about 1 of every 5000 newborns, have been characterized.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.