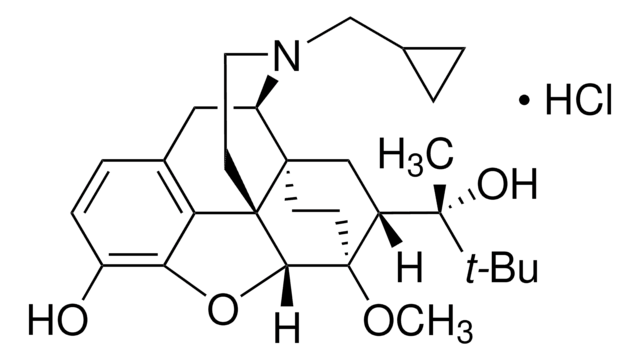
B-902
Buprenorphin -Lösung
100 μg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Empfohlene Produkte
Qualität
certified reference material
Qualitätsniveau
Form
liquid
Leistungsmerkmale
SNAP-N-SPIKE®, SNAP-N-SHOOT®
Verpackung
ampule of 1 mL
Hersteller/Markenname
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIC (Portugal)
Konzentration
100 μg/mL in methanol
Methode(n)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Anwendung(en)
forensics and toxicology
Format
single component solution
Lagertemp.
2-8°C
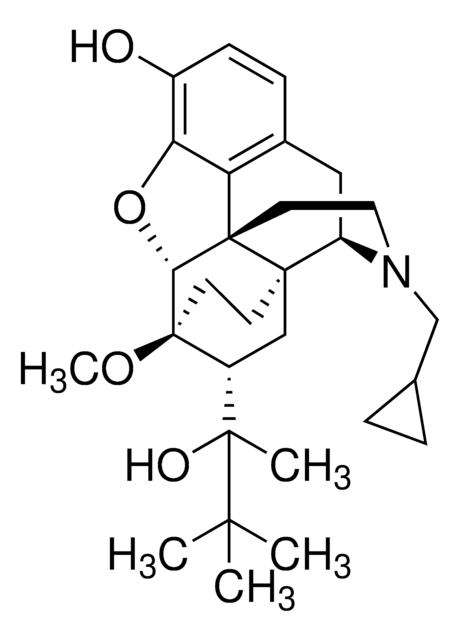
SMILES String
CO[C@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]4Cc5ccc(O)c6O[C@@H]2[C@]3(CCN4CC7CC7)c56
InChI
1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1
InChIKey
RMRJXGBAOAMLHD-IHFGGWKQSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Research on Addiction and Implementation Challenges: Buprenorphine solutions have been crucial in studies exploring how systemic barriers affect the implementation of addiction treatments during concurrent public health crises, offering insights valuable for policy and practice adjustments in healthcare systems (Sharp et al., 2023).
- Evaluating Patient-Important Measures in Opioid Use Disorder Treatments: Buprenorphine is used extensively to evaluate patient-important outcomes in medication-assisted treatment programs for opioid use disorders, aiding in the assessment of long-term recovery metrics and treatment efficacy (Reed et al., 2023).
- Web-Based Substance Use Epidemiology Research: Buprenorphine research solutions are instrumental in developing web-based ontologies for tracking and analyzing drug abuse trends, providing a foundational tool for public health surveillance and policy-making in substance abuse epidemiology (Lokala et al., 2022).
- Clinical Studies in Veterinary Medicine: Buprenorphine solutions are employed in veterinary medicine to assess the efficacy of transdermal applications for managing postoperative pain in animals, demonstrating its versatility and effectiveness across different biological systems (Clark et al., 2022).
- Pain Management in Geriatric Orthopedics: The use of transdermal buprenorphine for managing pain following femur fractures in elderly patients highlights its critical role in improving postoperative care and enhancing recovery outcomes in geriatric orthopedics (Davies et al., 2022).
Rechtliche Hinweise
Ähnliches Produkt
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
49.5 °F - closed cup
Flammpunkt (°C)
9.7 °C - closed cup
Zulassungslistungen
Zulassungslistungen werden hauptsächlich für chemische Produkte erstellt. Für nicht-chemische Produkte können hier nur begrenzte Angaben gemacht werden. Kein Eintrag bedeutet, dass keine der Komponenten gelistet ist. Es liegt in der Verantwortung des Benutzers, die sichere und legale Verwendung des Produkts zu gewährleisten.
EU REACH Annex XVII (Restriction List)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.