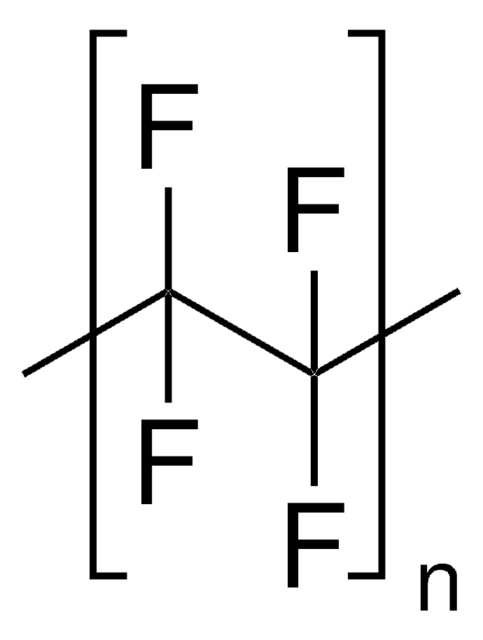
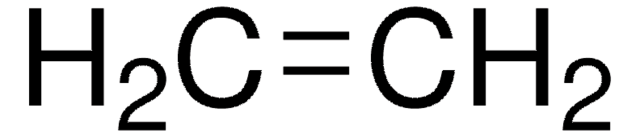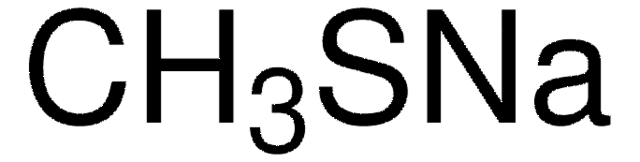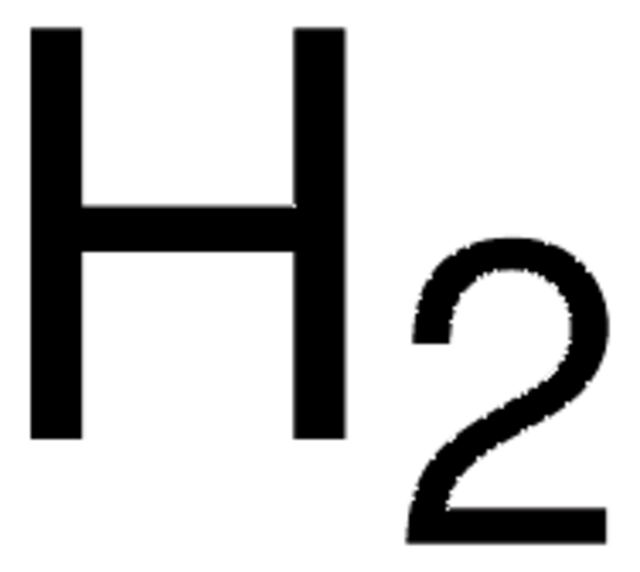536164
Ethylen
99.99%
Synonym(e):
Ethen
About This Item
Empfohlene Produkte
Dampfdichte
0.97 (vs air)
Qualitätsniveau
Dampfdruck
35.04 atm ( 20 °C)
Assay
99.99%
Selbstzündungstemp.
842 °F
Expl.-Gr.
36 %
bp
−104 °C (lit.)
mp (Schmelzpunkt)
−169 °C (lit.)
SMILES String
C=C
InChI
1S/C2H4/c1-2/h1-2H2
InChIKey
VGGSQFUCUMXWEO-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Verpackung
Sonstige Hinweise
Rechtliche Hinweise
Empfehlung
Empfohlenes Ventil
Entlüftungsventil
Regulierungsbehörde
Schlaucholive
auch häufig zusammen mit diesem Produkt gekauft
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Flam. Gas 1 - Press. Gas Liquefied gas - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
2A - Gases
WGK
nwg
Flammpunkt (°F)
-148.0 °F - closed cup
Flammpunkt (°C)
-100 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.