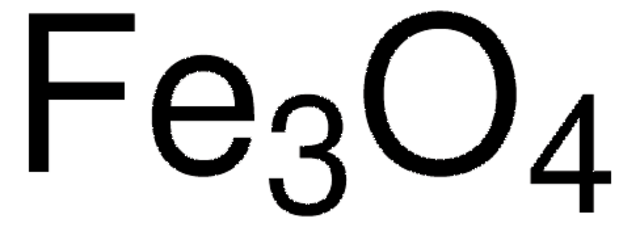518158
Eisen(II,III)-oxid
99.99% trace metals basis
Synonym(e):
Magnetit
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
99.99% trace metals basis
Form
powder
mp (Schmelzpunkt)
1538 °C (lit.)
Dichte
4.8-5.1 g/mL at 25 °C (lit.)
Anwendung(en)
battery manufacturing
SMILES String
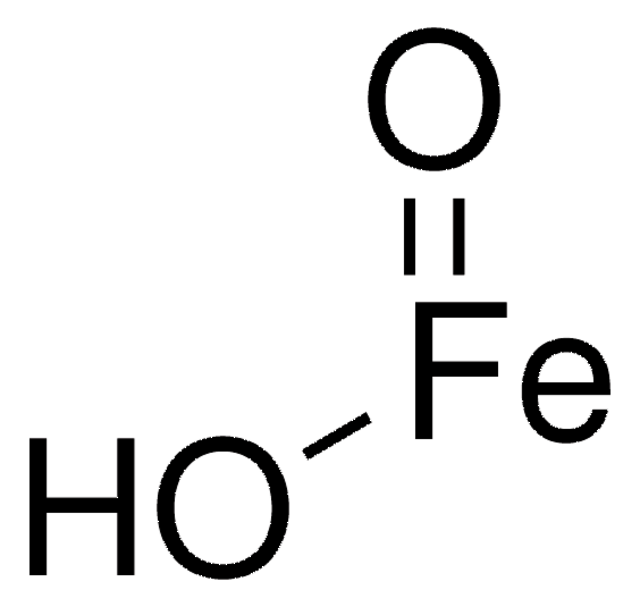
O=[Fe].O=[Fe]O[Fe]=O
InChI
1S/3Fe.4O
InChIKey
SZVJSHCCFOBDDC-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Achieving Excellent Dielectric and Energy Storage Performance in Core-Double-Shell-Structured Polyetherimide Nanocomposites.: This study explores the development of polyetherimide nanocomposites incorporating Iron(II,III) oxide for enhanced dielectric properties and energy storage capabilities, showing potential for advanced electrical applications (Yuan et al., 2023).
Lagerklassenschlüssel
11 - Combustible Solids
WGK
nwg
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
An article concerning self-propagating reactions induced by mechanical alloying, presented by Sigma-Aldrich.com.
Magnetic materials permeate numerous daily activities in our lives. They are essential components of a diversity of products including hard drives that reliably store information on our computers, decorative magnets that keep the shopping list attached to the refrigerator door, electric bicycles that speed our commute to work, as well as wind turbines for conversion of wind energy to electrical power.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.