357804
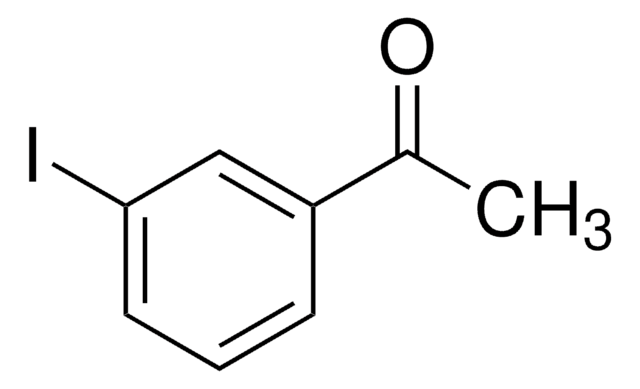
4′-Iodacetophenon
≥97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
IC6H4COCH3
CAS-Nummer:
Molekulargewicht:
246.05
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
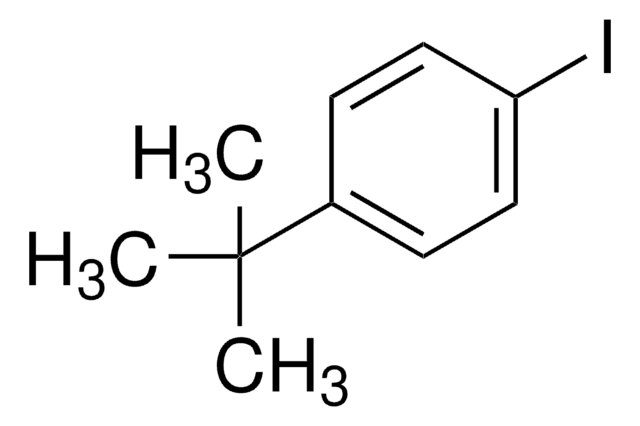
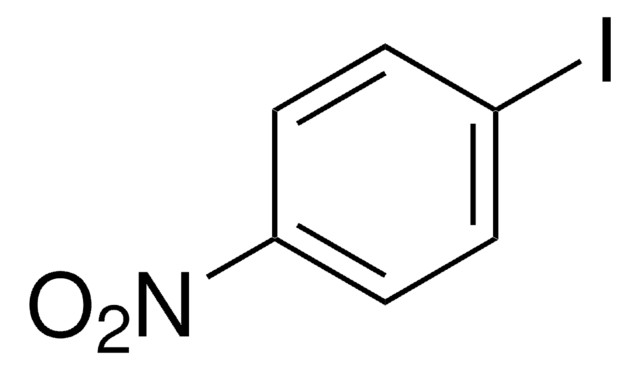
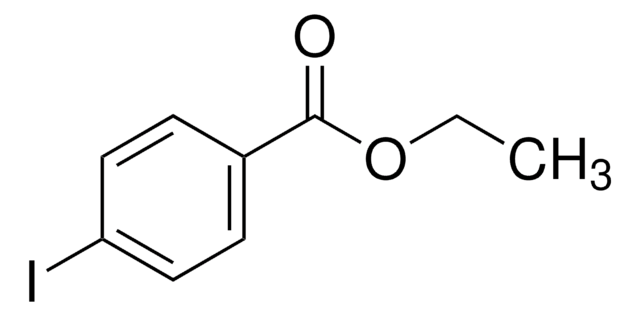
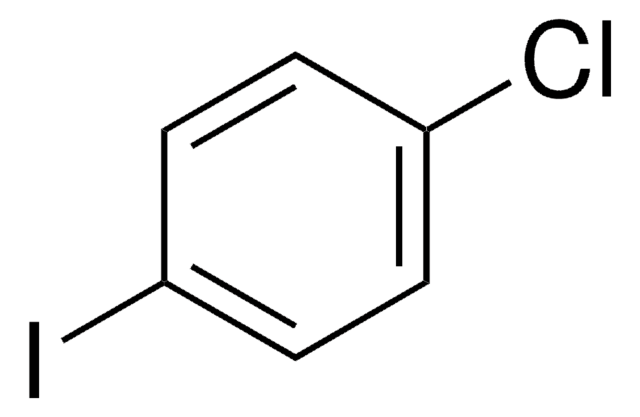
Empfohlene Produkte
Qualitätsniveau
Assay
≥97%
mp (Schmelzpunkt)
82-84 °C (lit.)
SMILES String
CC(C1=CC=C(I)C=C1)=O
InChI
1S/C8H7IO/c1-6(10)7-2-4-8(9)5-3-7/h2-5H,1H3
InChIKey
JZJWCDQGIPQBAO-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Pd(0)-catalyzed cross coupling reaction of 4′-iodoacetophenone with siloxane has been reported. Heck-Mizoroki reactions of 4′-iodoacetophenone with styrene catalyzed by Pd nanoparticles in the flow reactor has been reported.
Anwendung
Convenient substrate for palladium-catalyzed coupling reactions. Used in the synthesis of quinoline-based potential anticancer agents.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
G J Atwell et al.
Journal of medicinal chemistry, 32(2), 396-401 (1989-02-01)
A series of phenyl-substituted derivatives of the "minimal" DNA-intercalating agent N-[2-(dimethylamino)-ethyl]-2-phenylquinoline-8-carboxamide (1) have been synthesized and evaluated for in vivo antitumor activity, in a continuing search for active compounds of this class with the lowest possible DNA association constants. Substitution
S E Denmark et al.
Organic letters, 3(11), 1749-1752 (2001-06-19)
A sequential ring-closing metathesis/silicon-assisted cross-coupling sequence has been developed. Alkenyldimethylsilyl ethers of omega-unsaturated alcohols undergo facile ring closure with Schrock's catalyst to afford five-, six-, and seven-membered cycloalkenylsiloxanes bearing substituents on both alkenyl carbons. These siloxanes were highly effective coupling
Klaas Mennecke et al.
Beilstein journal of organic chemistry, 5, 21-21 (2009-07-11)
The preparation of monolithic polyionic supports which serve as efficient heterogeneous supports for palladium(0) nanoparticles is described. These functionalized polymers were incorporated inside a flow reactor and employed in Suzuki-Miyaura and Heck cross couplings under continuous flow conditions.
Chemistry Letters (Jpn), 2049-2049 (1989)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)